Abstract
The sequence of the pckA gene coding for phosphoenolpyruvate carboxykinase in Escherichia coli K-12 and previous molecular weight determinations indicate that this allosteric enzyme is a monomer of Mr 51,316. The protein is homologous to ATP-dependent phosphoenolpyruvate carboxykinases from Trypanosoma brucei and Saccharomyces cerevisiae. A potential ATP binding site was conserved in all three sequences. A potential binding site for the allosteric activator, calcium, identified in the E. coli enzyme, was only partially conserved in T. brucei and S. cerevisiae, consistent with the observation that the enzymes from the latter organisms were not activated by calcium. The published sequence of the ompR and envZ genes from Salmonella typhimurium is followed by a partial sequence that is highly homologous to pckA from E. coli. The order of these genes and the direction of transcription of the presumptive S. typhimurium pckA gene are the same as those in E. coli. The potential calcium binding site of the E. coli enzyme is conserved in the partial predicted sequence of the S. typhimurium phosphoenolpyruvate carboxykinase, consistent with the observation that calcium activation of the S. typhimurium phosphoenolpyruvate carboxykinase is very similar to that observed for the E. coli enzyme. A pckA mRNA transcript was observed in stationary-phase cells but not in logarithmically growing cells. The mRNA start site was mapped relative to the sequence of the pckA structural gene.
Full text
PDF


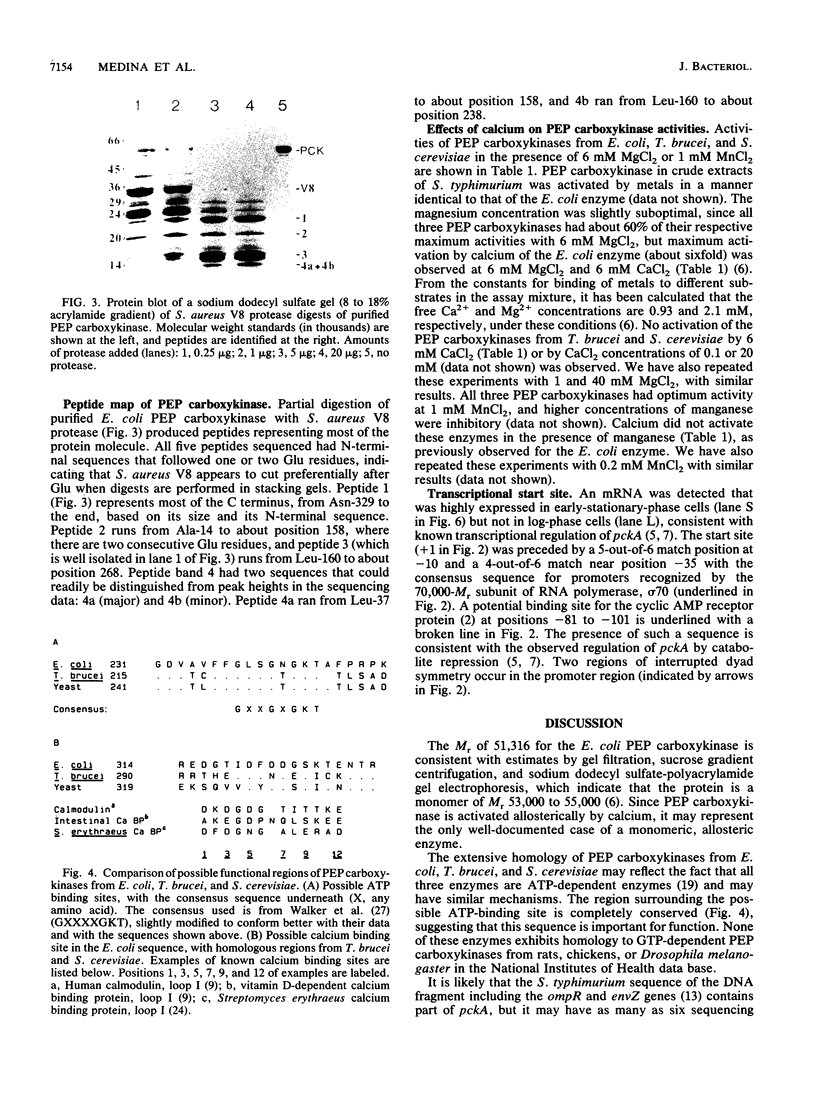
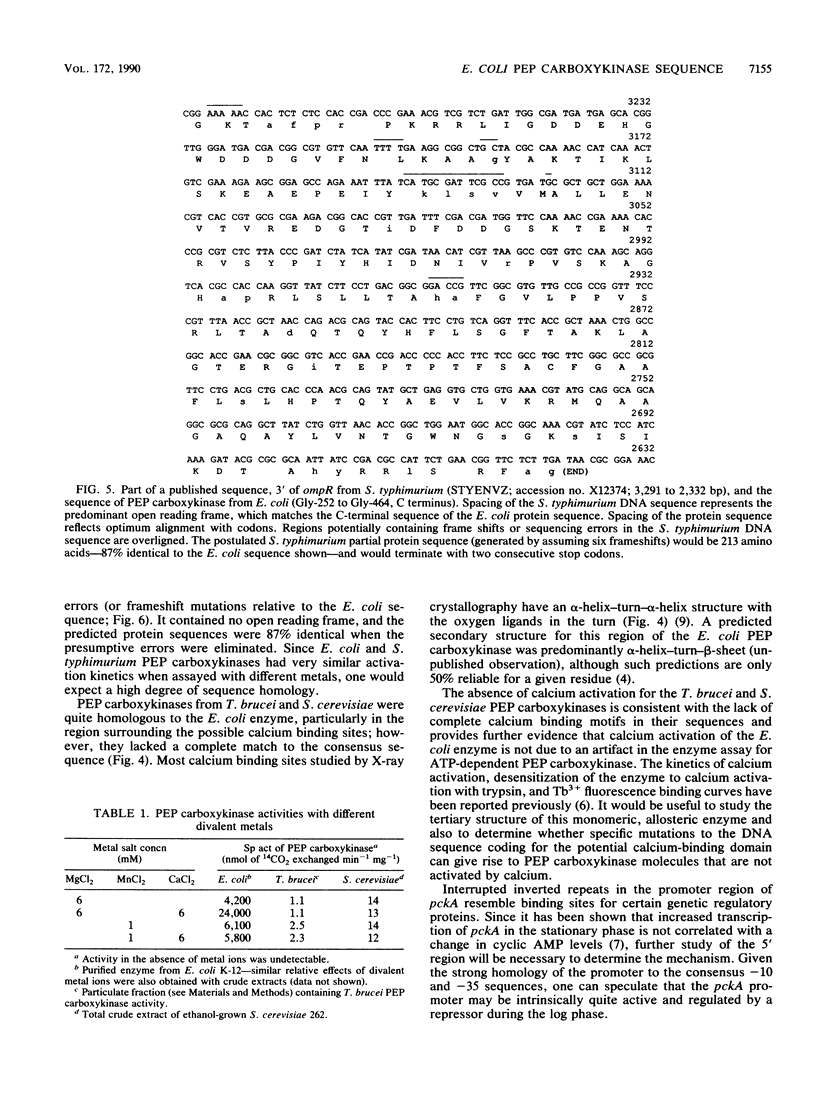

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M., Claverie-Martín F., Díaz-Torres M. R., Kushner S. R. Transcript mapping using [35S]DNA probes, trichloroacetate solvent and dideoxy sequencing ladders: a rapid method for identification of transcriptional start points. Gene. 1988 May 15;65(1):101–110. doi: 10.1016/0378-1119(88)90421-0. [DOI] [PubMed] [Google Scholar]
- Fischer S. G. Peptide mapping in gels. Methods Enzymol. 1983;100:424–430. doi: 10.1016/0076-6879(83)00071-3. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Allosteric control by calcium and mechanism of desensitization of phosphoenolpyruvate carboxykinase of Escherichia coli. J Biol Chem. 1980 Feb 25;255(4):1399–1405. [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Genetic and physiological characterization of Escherichia coli mutants deficient in phosphoenolpyruvate carboxykinase activity. J Bacteriol. 1980 Mar;141(3):1115–1121. doi: 10.1128/jb.141.3.1115-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie H., Medina V. Physical and genetic analysis of the phosphoenolpyruvate carboxykinase (pckA) locus from Escherichia coli K12. Mol Gen Genet. 1990 Jan;220(2):191–196. doi: 10.1007/BF00260481. [DOI] [PubMed] [Google Scholar]
- Goldie H. Regulation of transcription of the Escherichia coli phosphoenolpyruvate carboxykinase locus: studies with pck-lacZ operon fusions. J Bacteriol. 1984 Sep;159(3):832–836. doi: 10.1128/jb.159.3.832-836.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg O., James M. N. Common structural framework of the two Ca2+/Mg2+ binding loops of troponin C and other Ca2+ binding proteins. Biochemistry. 1985 Sep 24;24(20):5298–5302. doi: 10.1021/bi00341a004. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. A., Linstead D. J., Wheeler M. V. Carbon dioxide fixation in trypanosomatids. Parasitology. 1975 Aug;71(1):93–107. doi: 10.1017/s003118200005318x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Laamanen I., Palva E. T. Structure and expression of the ompB operon, the regulatory locus for the outer membrane porin regulon in Salmonella typhimurium LT-2. J Mol Biol. 1988 Jun 20;201(4):663–673. doi: 10.1016/0022-2836(88)90465-2. [DOI] [PubMed] [Google Scholar]
- Lloubes R., Baty D., Lazdunski C. The promoters of the genes for colicin production, release and immunity in the ColA plasmid: effects of convergent transcription and Lex A protein. Nucleic Acids Res. 1986 Mar 25;14(6):2621–2636. doi: 10.1093/nar/14.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal Biochem. 1986 Oct;158(1):165–170. doi: 10.1016/0003-2697(86)90605-6. [DOI] [PubMed] [Google Scholar]
- Parsons M., Smith J. M. Trypanosome glycosomal protein P60 is homologous to phosphoenolpyruvate carboxykinase (ATP). Nucleic Acids Res. 1989 Aug 11;17(15):6411–6411. doi: 10.1093/nar/17.15.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R., McLachlan A. D. Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res. 1982 Jan 11;10(1):141–156. doi: 10.1093/nar/10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucka R., Valdés-Hevia M. D., Gancedo C., Schwarzlose C., Feldmann H. Nucleotide sequence of the phosphoenolpyruvate carboxykinase gene from Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Nov 25;16(22):10926–10926. doi: 10.1093/nar/16.22.10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan D. G., Hale R. S., Dhillon N., Leadlay P. F. A bacterial calcium-binding protein homologous to calmodulin. Nature. 1987 Sep 3;329(6134):84–85. doi: 10.1038/329084a0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. J., Bhattacharjee J. K. Regulation of phosphoenolpyruvate carboxykinase and pyruvate kinase in Saccharomyces cerevisiae grown in the presence of glycolytic and gluconeogenic carbon sources and the role of mitochondrial function on gluconeogenesis. Can J Microbiol. 1986 Dec;32(12):969–972. doi: 10.1139/m86-180. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]