Abstract
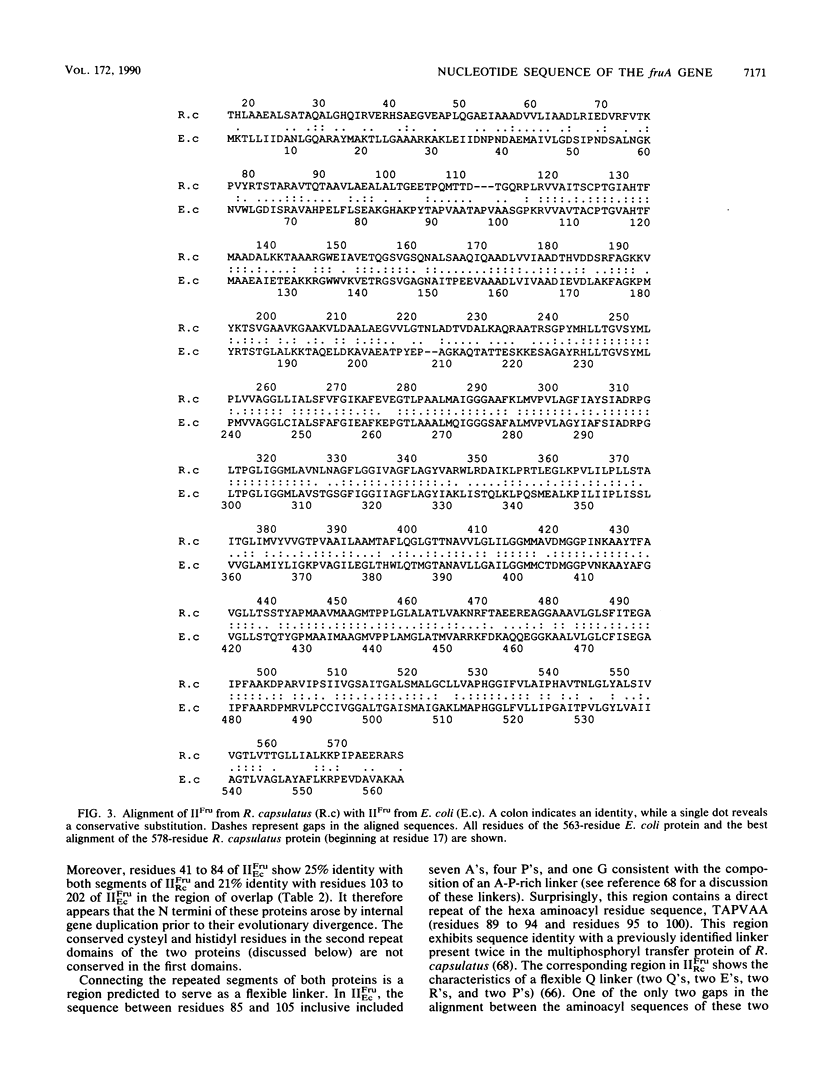
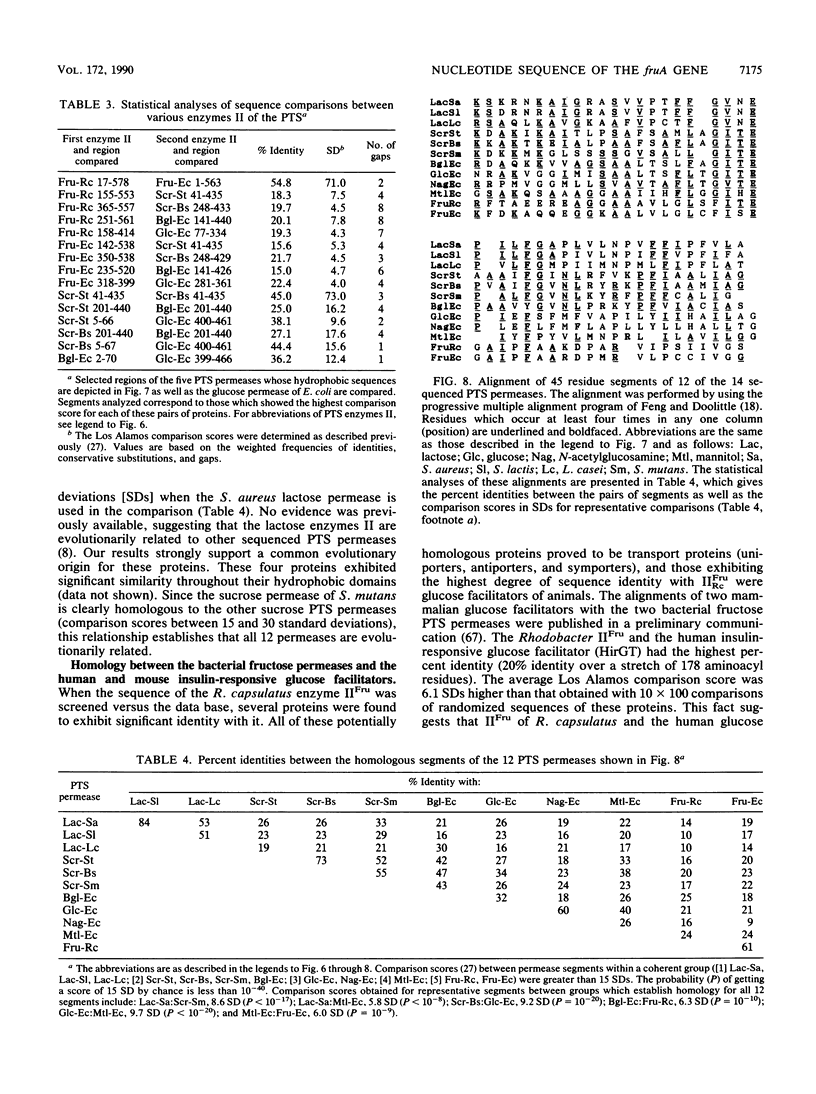
The nucleotide sequence of the fruA gene, the terminal gene in the fructose operon of Rhodobacter capsulatus, is reported. This gene codes for the fructose permease (molecular weight, 58,575; 578 aminoacyl residues), the fructose enzyme II (IIFru) of the phosphoenolpyruvate-dependent phosphotransferase system. The deduced aminoacyl sequence of the encoded gene product was found to be 55% identical throughout most of its length with the fructose enzyme II of Escherichia coli, with some regions strongly conserved and others weakly conserved. Sequence comparisons revealed that the first 100 aminoacyl residues of both enzymes II were homologous to the second 100 residues, suggesting that an intragenic duplication of about 300 nucleotides had occurred during the evolution of IIFru prior to divergence of the E. coli and R. capsulatus genes. The protein contains only two cysteyl residues, and only one of these residues is conserved between the two proteins. This residue is therefore presumed to provide the active-site thiol group which may serve as the phosphorylation site. IIFru was found to exhibit regions of homology with sequenced enzymes II from other bacteria, including those specific for sucrose, beta-glucosides, mannitol, glucose, N-acetylglucosamine, and lactose. The degree of evolutionary divergence differed for different parts of the proteins, with certain transmembrane segments exhibiting high degrees of conservation. The hydrophobic domain of IIFru was also found to be similar to several uniport and antiport transporters of animals, including the human and mouse insulin-responsive glucose facilitators. These observations suggest that the mechanism of transmembrane transport may be similar for permeases catalyzing group translocation and facilitated diffusion.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M. E., Saier M. H., Jr A common ancestor for bovine lens fiber major intrinsic protein, soybean nodulin-26 protein, and E. coli glycerol facilitator. Cell. 1990 Jan 26;60(2):185–186. doi: 10.1016/0092-8674(90)90731-s. [DOI] [PubMed] [Google Scholar]
- Bear D. G., Peabody D. S. The E. coli Rho protein: an ATPase that terminates transcription. Trends Biochem Sci. 1988 Sep;13(9):343–347. doi: 10.1016/0968-0004(88)90104-1. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley H. F., Kornberg H. L. Nucleotide sequence of bglC, the gene specifying enzymeIIbgl of the PEP:sugar phosphotransferase system in Escherichia coli K12, and overexpression of the gene product. J Gen Microbiol. 1987 Mar;133(3):563–573. doi: 10.1099/00221287-133-3-563. [DOI] [PubMed] [Google Scholar]
- Bramley H. F., Kornberg H. L. Sequence homologies between proteins of bacterial phosphoenolpyruvate-dependent sugar phosphotransferase systems: identification of possible phosphate-carrying histidine residues. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4777–4780. doi: 10.1073/pnas.84.14.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidt F., Jr, Hengstenberg W., Finkeldei U., Stewart G. C. Identification of the genes for the lactose-specific components of the phosphotransferase system in the lac operon of Staphylococcus aureus. J Biol Chem. 1987 Dec 5;262(34):16444–16449. [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Alpert C. A. Molecular characterization of the plasmid-encoded lactose-PTS of Lactobacillus casei. FEMS Microbiol Rev. 1989 Jun;5(1-2):157–165. doi: 10.1016/0168-6445(89)90020-x. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Lazarides E. Alternative primary structures in the transmembrane domain of the chicken erythroid anion transporter. Mol Cell Biol. 1988 Mar;8(3):1327–1335. doi: 10.1128/mcb.8.3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels G. A., Drews G., Saier M. H., Jr Properties of a Tn5 insertion mutant defective in the structural gene (fruA) of the fructose-specific phosphotransferase system of Rhodobacter capsulatus and cloning of the fru regulon. J Bacteriol. 1988 Apr;170(4):1698–1703. doi: 10.1128/jb.170.4.1698-1703.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner R., Lengeler J. W. DNA sequence of the gene scrA encoding the sucrose transport protein EnzymeII(Scr) of the phosphotransferase system from enteric bacteria: homology of the EnzymeII(Scr) and EnzymeII(Bgl) proteins. Mol Microbiol. 1988 Jan;2(1):9–17. [PubMed] [Google Scholar]
- Eiglmeier K., Boos W., Cole S. T. Nucleotide sequence and transcriptional startpoint of the glpT gene of Escherichia coli: extensive sequence homology of the glycerol-3-phosphate transport protein with components of the hexose-6-phosphate transport system. Mol Microbiol. 1987 Nov;1(3):251–258. doi: 10.1111/j.1365-2958.1987.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Erni B., Zanolari B. Glucose-permease of the bacterial phosphotransferase system. Gene cloning, overproduction, and amino acid sequence of enzyme IIGlc. J Biol Chem. 1986 Dec 15;261(35):16398–16403. [PubMed] [Google Scholar]
- Erni B., Zanolari B., Graff P., Kocher H. P. Mannose permease of Escherichia coli. Domain structure and function of the phosphorylating subunit. J Biol Chem. 1989 Nov 5;264(31):18733–18741. [PubMed] [Google Scholar]
- Erni B., Zanolari B., Kocher H. P. The mannose permease of Escherichia coli consists of three different proteins. Amino acid sequence and function in sugar transport, sugar phosphorylation, and penetration of phage lambda DNA. J Biol Chem. 1987 Apr 15;262(11):5238–5247. [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Fouet A., Arnaud M., Klier A., Rapoport G. Bacillus subtilis sucrose-specific enzyme II of the phosphotransferase system: expression in Escherichia coli and homology to enzymes II from enteric bacteria. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8773–8777. doi: 10.1073/pnas.84.24.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Geerse R. H., Izzo F., Postma P. W. The PEP: fructose phosphotransferase system in Salmonella typhimurium: FPr combines enzyme IIIFru and pseudo-HPr activities. Mol Gen Genet. 1989 Apr;216(2-3):517–525. doi: 10.1007/BF00334399. [DOI] [PubMed] [Google Scholar]
- Grisafi P. L., Scholle A., Sugiyama J., Briggs C., Jacobson G. R., Lengeler J. W. Deletion mutants of the Escherichia coli K-12 mannitol permease: dissection of transport-phosphorylation, phospho-exchange, and mannitol-binding activities. J Bacteriol. 1989 May;171(5):2719–2727. doi: 10.1128/jb.171.5.2719-2727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J. The homologous glucose transport proteins of prokaryotes and eukaryotes. Res Microbiol. 1990 Mar-Apr;141(3):316–328. doi: 10.1016/0923-2508(90)90005-b. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. The role of ATP in binding-protein-dependent transport systems. Res Microbiol. 1990 Mar-Apr;141(3):353–360. doi: 10.1016/0923-2508(90)90011-e. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M. I. Los Alamos sequence analysis package for nucleic acids and proteins. Nucleic Acids Res. 1982 Jan 11;10(1):183–196. doi: 10.1093/nar/10.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lolkema J. S., Robillard G. T. Phosphoenolpyruvate-dependent fructose phosphotransferase system in Rhodopseudomonas sphaeroides. The coupling between transport and phosphorylation in inside-out vesicles. Eur J Biochem. 1985 Feb 15;147(1):69–75. doi: 10.1111/j.1432-1033.1985.tb08720.x. [DOI] [PubMed] [Google Scholar]
- Lolkema J. S., ten Hoeve-Duurkens R. H., Robillard G. T. The phosphoenolpyruvate-dependent fructose-specific phosphotransferase system in Rhodopseudomonas sphaeroides. Energetics of the phosphoryl group transfer from phosphoenolpyruvate to fructose. Eur J Biochem. 1986 Jan 15;154(2):387–393. doi: 10.1111/j.1432-1033.1986.tb09410.x. [DOI] [PubMed] [Google Scholar]
- Lolkema J. S., ten Hoeve-Duurkens R. H., Robillard G. T. The phosphoenolpyruvate-dependent fructose-specific phosphotransferase system in Rhodopseudomonas sphaeroides. Mechanism for transfer of the phosphoryl group from phosphoenolpyruvate to fructose. Eur J Biochem. 1985 Jun 18;149(3):625–631. doi: 10.1111/j.1432-1033.1985.tb08970.x. [DOI] [PubMed] [Google Scholar]
- Maloney P. C. A consensus structure for membrane transport. Res Microbiol. 1990 Mar-Apr;141(3):374–383. doi: 10.1016/0923-2508(90)90015-i. [DOI] [PubMed] [Google Scholar]
- Manayan R., Tenn G., Yee H. B., Desai J. D., Yamada M., Saier M. H., Jr Genetic analyses of the mannitol permease of Escherichia coli: isolation and characterization of a transport-deficient mutant which retains phosphorylation activity. J Bacteriol. 1988 Mar;170(3):1290–1296. doi: 10.1128/jb.170.3.1290-1296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Osmochemistry of solute translocation. Res Microbiol. 1990 Mar-Apr;141(3):286–289. doi: 10.1016/0923-2508(90)90002-8. [DOI] [PubMed] [Google Scholar]
- Nuoffer C., Zanolari B., Erni B. Glucose permease of Escherichia coli. The effect of cysteine to serine mutations on the function, stability, and regulation of transport and phosphorylation. J Biol Chem. 1988 May 15;263(14):6647–6655. [PubMed] [Google Scholar]
- Pas H. H., Robillard G. T. Enzyme IIMtl of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: identification of the activity-linked cysteine on the mannitol carrier. Biochemistry. 1988 Jul 26;27(15):5515–5519. doi: 10.1021/bi00415a019. [DOI] [PubMed] [Google Scholar]
- Peri K. G., Waygood E. B. Sequence of cloned enzyme IIN-acetylglucosamine of the phosphoenolpyruvate:N-acetylglucosamine phosphotransferase system of Escherichia coli. Biochemistry. 1988 Aug 9;27(16):6054–6061. doi: 10.1021/bi00416a034. [DOI] [PubMed] [Google Scholar]
- Postma P. W. Defective enzyme II-BGlc of the phosphoenolpyruvate:sugar phosphotransferase system leading to uncoupling of transport and phosphorylation in Salmonella typhimurium. J Bacteriol. 1981 Aug;147(2):382–389. doi: 10.1128/jb.147.2.382-389.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior T. I., Kornberg H. L. Nucleotide sequence of fruA, the gene specifying enzyme IIfru of the phosphoenolpyruvate-dependent sugar phosphotransferase system in Escherichia coli K12. J Gen Microbiol. 1988 Oct;134(10):2757–2768. doi: 10.1099/00221287-134-10-2757. [DOI] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr Involvement of lactose enzyme II of the phosphotransferase system in rapid expulsion of free galactosides from Streptococcus pyogenes. J Bacteriol. 1983 Oct;156(1):236–242. doi: 10.1128/jb.156.1.236-242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Ohgi T., Plumbridge J., Söll D. Nucleotide sequences of the Escherichia coli nagE and nagB genes: the structural genes for the N-acetylglucosamine transport protein of the bacterial phosphoenolpyruvate: sugar phosphotransferase system and for glucosamine-6-phosphate deaminase. Gene. 1988;62(2):197–207. doi: 10.1016/0378-1119(88)90558-6. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Evolution of permease diversity and energy-coupling mechanisms: an introduction. Res Microbiol. 1990 Mar-Apr;141(3):281–286. doi: 10.1016/0923-2508(90)90001-7. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Roseman S. Phosphoenolpyruvate-dependent fructose phosphorylation in photosynthetic bacteria. J Biol Chem. 1971 Dec 25;246(24):7819–7821. [PubMed] [Google Scholar]
- Saier M. H., Jr, Grenier F. C., Lee C. A., Waygood E. B. Evidence for the evolutionary relatedness of the proteins of the bacterial phosphoenolpyruvate:sugar phosphotransferase system. J Cell Biochem. 1985;27(1):43–56. doi: 10.1002/jcb.240270106. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Werner P. K., Müller M. Insertion of proteins into bacterial membranes: mechanism, characteristics, and comparisons with the eucaryotic process. Microbiol Rev. 1989 Sep;53(3):333–366. doi: 10.1128/mr.53.3.333-366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Yamada M., Erni B., Suda K., Lengeler J., Ebner R., Argos P., Rak B., Schnetz K., Lee C. A. Sugar permeases of the bacterial phosphoenolpyruvate-dependent phosphotransferase system: sequence comparisons. FASEB J. 1988 Mar 1;2(3):199–208. doi: 10.1096/fasebj.2.3.2832233. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sato Y., Poy F., Jacobson G. R., Kuramitsu H. K. Characterization and sequence analysis of the scrA gene encoding enzyme IIScr of the Streptococcus mutans phosphoenolpyruvate-dependent sucrose phosphotransferase system. J Bacteriol. 1989 Jan;171(1):263–271. doi: 10.1128/jb.171.1.263-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz K., Sutrina S. L., Saier M. H., Jr, Rak B. Identification of catalytic residues in the beta-glucoside permease of Escherichia coli by site-specific mutagenesis and demonstration of interdomain cross-reactivity between the beta-glucoside and glucose systems. J Biol Chem. 1990 Aug 15;265(23):13464–13471. [PubMed] [Google Scholar]
- Schnetz K., Toloczyki C., Rak B. Beta-glucoside (bgl) operon of Escherichia coli K-12: nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J Bacteriol. 1987 Jun;169(6):2579–2590. doi: 10.1128/jb.169.6.2579-2590.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Greeb J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem. 1988 Jun 25;263(18):8646–8657. [PubMed] [Google Scholar]
- Solomon E., Miyal K., Lin E. C. Membrane translocation of mannitol in Escherichia coli without phosphorylation. J Bacteriol. 1973 May;114(2):723–728. doi: 10.1128/jb.114.2.723-728.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrina S. L., Reizer J., Saier M. H., Jr Inducer expulsion in Streptococcus pyogenes: properties and mechanism of the efflux reaction. J Bacteriol. 1988 Apr;170(4):1874–1877. doi: 10.1128/jb.170.4.1874-1877.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkutnicka K., Tschopp J. F., Andrews L., Cirillo V. P. Sequence and structure of the yeast galactose transporter. J Bacteriol. 1989 Aug;171(8):4486–4493. doi: 10.1128/jb.171.8.4486-4493.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton J. C., Drummond M. H. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. 1989 May;2(7):535–543. doi: 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]
- Wu L. F., Saier M. H., Jr On the evolutionary origins of the bacterial phosphoenolpyruvate:sugar phosphotransferase system. Mol Microbiol. 1990 Jul;4(7):1219–1222. doi: 10.1111/j.1365-2958.1990.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Wu L. F., Tomich J. M., Saier M. H., Jr Structure and evolution of a multidomain multiphosphoryl transfer protein. Nucleotide sequence of the fruB(HI) gene in Rhodobacter capsulatus and comparisons with homologous genes from other organisms. J Mol Biol. 1990 Jun 20;213(4):687–703. doi: 10.1016/S0022-2836(05)80256-6. [DOI] [PubMed] [Google Scholar]
- Yamada M., Saier M. H., Jr Glucitol-specific enzymes of the phosphotransferase system in Escherichia coli. Nucleotide sequence of the gut operon. J Biol Chem. 1987 Apr 25;262(12):5455–5463. [PubMed] [Google Scholar]



