Abstract
Estradiol-17β (E2) acts through the estrogen receptor (ER) to regulate uterine growth and functional differentiation. To determine whether E2 elicits epithelial mitogenesis through epithelial ER versus indirectly via ER-positive stromal cells, uteri from adult ER-deficient ER knockout (ko) mice and neonatal ER-positive wild-type (wt) BALB/c mice were used to produce the following tissue recombinants containing ER in epithelium (E) and/or stroma (S), or lacking ER altogether: wt-S + wt-E, wt-S + ko-E, ko-S + ko-E, and ko-S + wt-E. Tissue recombinants were grown for 4 weeks as subrenal capsule grafts in intact female nude mice, then the hosts were treated with either E2 or oil a week after ovariectomy. Epithelial labeling index and ER expression were determined by [3H]thymidine autoradiography and immunohistochemistry, respectively. In tissue recombinants containing wt-S (wt-S + wt-E, wt-S + ko-E), E2 induced a similar large increase in epithelial labeling index compared with oil-treated controls in both types of tissue recombinants despite the absence of epithelial ER in wt-S + ko-E tissue recombinants. This proliferative effect was blocked by an ER antagonist, indicating it was mediated through ER. In contrast, in tissue recombinants prepared with ko-S (ko-S + ko-E and ko-S + wt-E), epithelial labeling index was low and not stimulated by E2 despite epithelial ER expression in ko-S + wt-E grafts. In conclusion, these data demonstrate that epithelial ER is neither necessary nor sufficient for E2-induced uterine epithelial proliferation. Instead, E2 induction of epithelial proliferation appears to be a paracrine event mediated by ER-positive stroma. These data in the uterus and similar studies in the prostate suggest that epithelial mitogenesis in both estrogen and androgen target organs are stromally mediated events.
Estradiol-17β (E2) stimulates uterine epithelial proliferation in vivo and is obligatory for normal uterine epithelial morphogenesis, cytodifferentiation, and secretory activity. E2 elicits its effects via estrogen receptors (ER). These receptors are expressed in both epithelial and stromal cells of juvenile and adult uterus (1–4), suggesting that the myriad effects of E2 on epithelium and stroma are mediated directly through ER in these tissue compartments. However, analysis of ER expression and E2 responsiveness in the developing uterus has indicated that this may be an oversimplification. Cunha et al. (5) used steroid autoradiography (ARG) to demonstrate that ER are undetectable in uterine epithelium (UtE) of neonatal BALB/c mice, but are present in the mesenchyme/stroma. Subsequent immunohistochemical studies confirmed that ER are undetectable in UtE from neonatal mice (1, 6, 7).
Despite the apparent lack of epithelial ER in the neonatal murine uterus, estrogen treatment caused a doubling in the rate of uterine epithelial proliferation, even though ER remained undetectable in neonatal UtE even after estrogen stimulation (8). Thus, mitogenic effects of E2 on neonatal UtE may be indirectly mediated by ER in the mesenchymal/stromal cells.
This interpretation is supported by Yamashita et al. (9), who used double-labeling studies to simultaneously examine ER expression and proliferation in neonatal UtE. They found that UtE of CD-1 mice contained both ER-positive and ER-negative cells at 4 days postpartum. Estrogen treatment strongly increased the percentage of epithelial cells that were ER-positive. However, estrogen-stimulated epithelial proliferation was similar in both ER-positive and ER-negative epithelial cells, again suggesting that E2 stimulated epithelial mitogenesis indirectly through stromal ER.
These in vivo studies are corroborated by in vitro data demonstrating that E2 is not mitogenic for isolated UtE cells in vitro (10–12). However, when cultured UtE is recombined with uterine stroma (UtS) and grafted in vivo, E2 again stimulated mitogenesis in UtE of these tissue recombinants (13). Furthermore, in cocultures of UtS and UtE, E2 increased epithelial DNA content, an effect not observed with E2 treatment of pure epithelial cultures (14). This further suggests that E2-induced epithelial mitogenesis is mediated indirectly via stroma.
Another interpretation is that despite the apparent lack of ER in neonatal mouse UtE, these epithelial cells could be expressing ER at levels below the limit of detection by present techniques. Such low levels of epithelial ER may be capable of directly mediating mitogenic effects of E2 (9, 15). Therefore, to definitively prove or disprove the hypothesis that E2 stimulates epithelial mitogenesis indirectly through the mesenchyme/stroma, another experimental approach is needed that will yield definitive data.
A critical advance in this field has been development of an estrogen receptor knockout (ERKO) mouse, in which the ER gene has been rendered nonfunctional by gene targeting (16–18). The ERKO mouse provides the unique opportunity to use tissue separation and recombination techniques to produce uterine tissue recombinants that lack ER in their epithelium, mesenchyme/stroma, or both.
The objective of this study was to use uterine tissue from adult ERKO and normal neonatal mice in conjunction with tissue recombination techniques to directly determine whether E2-induced epithelial proliferation can occur in absence of epithelial ER. Previous work has indicated that mitogenic effects of androgen on male reproductive organs were mediated indirectly through the stroma. The present experiments provided an opportunity to determine if this process is unique to androgen or also occurs with other sex steroids. Our results indicate that uterine epithelial ER is neither necessary nor sufficient for E2-induced epithelial mitogenesis, and strongly suggest that E2-induced epithelial proliferation is mediated through mesenchymal/stromal ER.
MATERIALS AND METHODS
Animals and Treatments.
Mice were maintained under controlled temperature and lighting conditions during the experiment, and were given food and water ad libitum.
ERKO mice were produced as described previously (16). Genotypes of pups were determined by multiplex PCR (16), and only homozygous ERKO females of a mixed C57BL6/129SV background were used in these experiments. Mid-pregnant BALB/c mice were purchased from Harlan Breeders (Indianapolis) or Bantin & Kingman (Fremont, CA), and uteri were obtained from newborn female pups.
Tissue Separation/Recombination, Grafting, and ARG.
Uteri were removed from adult (90–120 day) ERKO and neonatal (0- to 3-day) BALB/c mice after sacrifice. To ensure that all homozygous ERKO mice were correctly identified, presence of hypoplastic uteri and hyperemic ovaries without corpora lutea typical of this mutation (16) were verified during dissection. Uteri from neonatal and adult mice were dissected free of adherent connective tissue and fat, placed into Hanks’ balanced salt solution, and cut into small pieces for trypsinization (19).
Procedures for separation of epithelium and stroma from uteri of mice have been described previously (19). Briefly, uteri from BALB/c and ERKO mice were enzymatically dissociated in a solution of 1% trypsin (Difco) in calcium- and magnesium-free Hanks’ balanced salt solution for 90 min at 4°C followed by gentle mechanical manipulation (19).
The following tissue recombinations were prepared by culturing recombined stroma and epithelium on agar plates overnight (20, 21): (1) wild type-stroma (wt-S) + wt-epithelium (wt-E); (2) wt-S + knockout-E (ko-E); (3) ko-S + wt-E, and (4) ko-S + ko-E. An n of at least 12 was used for all tissue recombinants, and data represent results of at least four separate experiments performed in two different laboratories.
Grafts were transplanted under renal capsules of intact adult (8- to 12-week-old) female nude mice (Harlan or Bantin & Kingman), as described previously (20, 21). Grafts were grown for approximately 1 month, and then all hosts were ovariectomized. Seven days later, some hosts were given 100 ng of E2 (Sigma) in 0.05 ml of corn oil i.p., while others were given vehicle alone. To determine if stimulation of epithelial proliferation seen in wt-S + wt-E and wt-S + ko-E tissue recombinants in response to E2 was mediated through ER, some hosts were given daily subcutaneous injections of 1 mg/kg of the anti-estrogen ICI 182,780 (ICI) in oil on days 5–7 post-ovariectomy, and then given E2 on day 7 post-ovariectomy.
Sixteen hours after E2 or oil treatments, all hosts were injected IP with [3H]thymidine (specific activity = 80 Ci/mmol; 1 Ci = 37 GBq; Amersham) at a dose of 2 μCi/g body weight. Two hr later (18 hr after E2 or oil injection), hosts were killed, and grafts were removed and fixed in neutral buffered formalin. Tissue recombinants were processed into paraffin and sectioned at 6 μm. Histological sections were immunohistochemically stained for ER (see below), then images of these sections were captured using a Leaf Lumina camera/scanner (Leaf Systems, Southborough, MA) interfaced to a Power Macintosh 8100/80 computer.
For [3H]thymidine ARG, mounted tissue sections were deparaffinized, dried, dipped in Kodak NTB-2 nuclear emulsion, and stored at 4°C for 3–4 weeks until sufficient labeling could be detected. Autoradiograms were developed by standard techniques (13), and slides were stained with hematoxylin and eosin. Epithelial labeling index in various tissue recombinants was measured as [3H]thymidine-labeled cells per total cells as described previously (21). Each point is based on analysis of at least three specimens. For each group, a minimum of 3,000 epithelial cells were scored. Data on epithelial proliferation in various groups were analyzed by Student’s t test, and means were considered different when P ≤ 0.05.
Immunohistochemistry for ER.
To immunohistochemically detect ER in mouse uterine tissue recombinants, an antigen retrieval method (22) was used on formalin-fixed paraffin sections. A peroxidase blocking solution (Pierce) was used to inactivate endogenous peroxidase, then avidin and biotin blocking solutions (Dako) were applied. Nonspecific binding was blocked using Super Block (Pierce). Slides were incubated with either primary antibody (anti-ER LH2; Novocastra) or a control nonspecific IgG (Dako) overnight at 4°C, washed, and then the secondary biotinylated anti-mouse antibody (Dako) was applied. After application of streptavidin conjugated to horseradish peroxidase (Dako), diaminobenzidine (Dako) was used as the chromogen.
To more clearly show the relationship between epithelial ER status and E2-induced proliferation, some wt-S + wt-E and wt-S + ko-E tissue recombinants from E2-injected hosts were first stained for ER. Immunohistochemical images were then captured using a Leaf Lumina camera/scanner interfaced to a Macintosh computer. After imaging, coverslips were removed, and slides were processed for [3H]thymidine ARG, as above. Silver grains from [3H]thymidine autoradiograms were imaged and superimposed on the original ER immunohistochemical images.
RESULTS
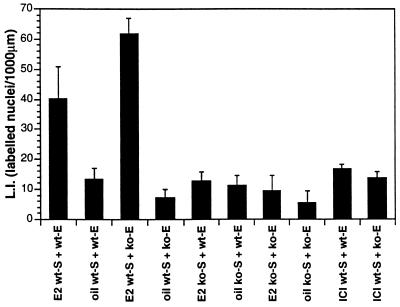
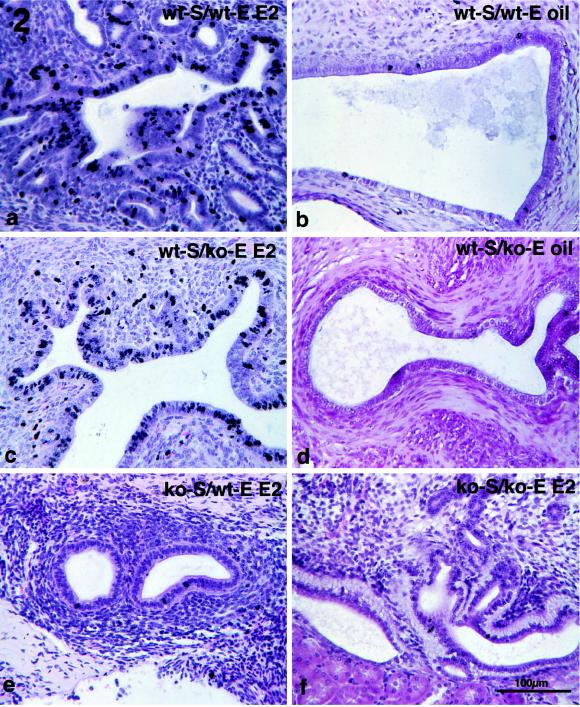
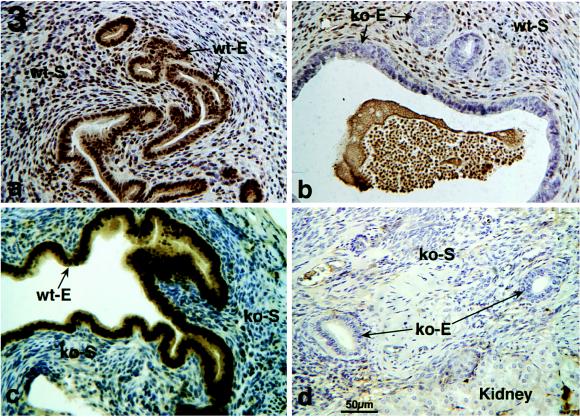
Thymidine ARG of various types of tissue recombinants indicated that an ER-negative epithelium can respond mitogenically to E2 when associated with ER-positive stroma. Epithelial labeling index in tissue recombinants composed of wt-S + wt-E and wt-S + ko-E was significantly increased (P ≤ 0.05) in E2− versus oil-treated specimens (Figs. 1 and 2 a-d). In contrast, in ko-S + wt-E and ko-S + ko-E tissue recombinants [3H]thymidine epithelial labeling index was low and showed no statistical difference in E2- versus oil-treated specimens (Figs. 1 and 2 e and f). Therefore, E2 does not stimulate epithelial proliferation in tissue recombinants lacking stromal ER, even when epithelial ER are present. Treatment with the ER antagonist ICI 182,780 blocked the proliferative effects of E2 (Fig. 1), indicating that the mitogenic effects of E2 were mediated through ER. As expected, both epithelium and stroma in wt-S + wt-E tissue recombinants exhibited intense nuclear staining with anti-ER antibody (Fig. 3a); sections treated with a control nonspecific IgG instead of the primary antibody exhibited only background staining (not shown). Anti-ER staining was nuclear in both stroma and epithelium. Intense nuclear staining was seen in stroma of wt-S + ko-E tissue recombinants, but epithelium was unstained (Fig. 3b). In tissue recombinants consisting of ko-S + wt-E, nuclear staining was observed only in epithelium (Fig. 3c), whereas both stromal and epithelial cells were unstained in tissue recombinants consisting of ko-S + ko-E (Fig. 3d). The ko-S + ko-E tissue recombinants function as an effective control for nonspecific background staining because all tissues are ER-negative. Background staining with the ER antibody was minimal and entirely cytoplasmic, as shown by weak diffuse light brown staining in cytoplasm of some cells (Fig. 3c).
Figure 1.
Labeling index of epithelium in uterine tissue recombinants (wt-S + wt-E, wt-S + ko-E, ko-S + wt-E and ko-S + ko-E). Grafts were grown for 1 month in female nude mouse hosts, and then hosts were ovariectomized. One week later, hosts were injected with either 100 ng of E2 or vehicle alone. One group of hosts receiving E2 also was treated with the anti-estrogen ICI 182,780 (1 mg/kg) on days 5–7 post-ovariectomy.
Figure 2.
Thymidine ARG of tissue recombinants consisting of wt-S + wt-E (a and b), wt-S + ko-E (c and d), ko-S + wt-E (e), and ko-S + ko-E (f). Grafts were grown for 1 month in female nude mouse hosts, and then hosts were ovariectomized and injected 1 week later with either 100 ng of E2 (a, c, e, and f) or oil vehicle (b and d).
Figure 3.
Immunohistochemical localization of ER in various types of tissue recombinants.
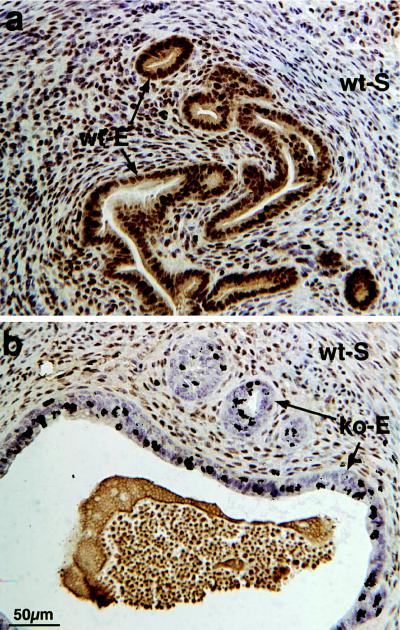
Simultaneous assessment of epithelial cell ER status and proliferation by using computer image analysis to superimpose ER immunohistochemical images with subsequent [3H]-thymidine autoradiographic images (Fig. 4) revealed that epithelial cells proliferating intensely in response to E2 were ER-positive in wt-S + wt-E tissue recombinants, as expected. In wt-S + ko-E tissue recombinants (Fig. 4), estrogen-induced epithelial proliferation ([3H]thymidine labeling) occurred in ER-negative epithelial cells.
Figure 4.
Simultaneous assessment of ER status and [3H]thymidine labeling in wt-S + wt-E (a) and wt-S + ko-E (b) tissue recombinants from ovariectomized hosts injected with E2.
DISCUSSION
Use of tissue separation/recombination techniques, in conjunction with wt ER-positive BALB/c and ER-negative ERKO uterine tissues used in this study, provide a powerful method for controlling ER status of both stroma and epithelium. By analyzing the effects of a lack of stromal and/or epithelial ER on a particular E2 response such as epithelial mitogenesis, the role of ER in each tissue compartment can be definitively determined.
Correlation between ER expression in UtE of juvenile and adult mice and well-known effects of E2 on epithelial mitogenesis led historically to the presumption that estrogenic effects were mediated directly through epithelial ER. However, a direct E2 effect on mitogenesis in either UtE or other female reproductive epithelia has never been conclusively demonstrated. Moreover, evidence from some studies in neonatal mice (8) and the lack of mitogenic effects of E2 on cultured UtE (10–12) suggested that E2 might act indirectly via stroma to promote uterine epithelial mitogenesis. However, definitive proof of this idea was lacking and plausible alternative explanations existed. For example, lack of a mitogenic effect of E2 on UtE in vitro could reflect insufficiencies in culture conditions that masked E2 mitogenicity. Similarly, mitogenic effects of estrogen in apparently ER-negative neonatal UtE (1, 6–8) could have resulted from epithelial ER expression below detectable levels but still sufficient to mediate a direct mitogenic response (15).
The present data strongly support the concept that the mitogenic response of UtE to E2 is mediated by stromal ER. The similar increase in magnitude of epithelial thymidine labeling in wt-S + wt-E and wt-S + ko-E tissue recombinants in response to E2 indicates that E2 stimulates comparable increases in epithelial mitogenesis in these two types of tissue recombinations. Immunohistochemical staining of ER in tissue recombinants provides a direct means of confirming ER status and origin of epithelium and stroma, and thus functions as an important control for verifying completeness of tissue separation techniques. Immunohistochemical staining of wt-S + ko-E tissue recombinants clearly indicates that epithelium in these tissue recombinants is derived from estrogen-insensitive ERKO mice and does not express functional ER. Lack of epithelial proliferation in response to E2 in ko-S + wt-E tissue recombinants, which contain an ER-positive epithelium, indicates that typical changes in epithelial proliferation induced by E2 require an ER-positive stroma and that epithelial ER alone are neither necessary nor sufficient for uterine epithelial mitogenic response to E2.
ICI 182,780 is a potent anti-estrogen that specifically blocks estrogen action by competing with E2 for binding to ER (23). The ability of ICI 182,780 to completely abolish proliferative effects of E2 on UtE in wt-S + ko-E tissue recombinants further suggests that the proliferative effect of E2 on epithelium is a paracrine event mediated through stromal ER rather than through interaction of E2 with another receptor, and most importantly indicates other nonreceptor-mediated actions of E2 are not involved.
Uterine epithelial ER in BALB/c mice are normally expressed beginning at about 4–5 days postnatal (8). Therefore, 0- to 3-day-old UtE used in these tissue recombination experiments lacked detectable ER when tissue recombinations were prepared and grafted. Expression of ER was expected in epithelium of wt-S + wt-E tissue recombinations at the time of harvest of grafts. However, expression of ER in epithelium of ko-S + wt-E tissue recombinants indicates that wt UtE can express ER even when recombined with UtS that lacks ER. Thus, ER-negative UtS from the ERKO uterus would appear to be permissive for expression of uterine epithelial ER. It should be noted, however, that the UtE was recombined with ko-S only a few days before epithelial ER expression. Therefore, commitment to express epithelial ER may have already been determined at the time of tissue recombination, and wt-E may simply be completing a developmental event that was initiated by stromal induction before tissue separation/recombination.
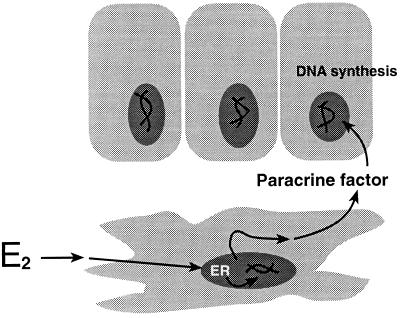
Based on earlier findings and present results, we propose the following model to explain estrogenic stimulation of uterine epithelial mitogenesis (Fig. 5). Estrogens bind to ER in uterine mesenchymal/stromal cells and trigger production of paracrine factors, which then act on UtE to stimulate mitogenesis. Although the present study focused exclusively on UtE, our preliminary results indicate that E2-induced mitogenesis also occurs by the same mechanism in vagina (unpublished data). Other types of E2 effects may be elicited either via the epithelial ER directly or may require the presence of both epithelial and stromal ER. Preliminary studies on E2 induction of the secretory protein, lactotransferrin, by UtE indicates that both stromal and epithelial ER are required for production of lactotransferrin protein and its mRNA in response to E2.
Figure 5.
Proposed mechanism of E2-stimulated epithelial proliferation in the uterus. Based on mitogenic response of ER-negative UtE to E2 when associated with ER-positive UtS and the ability of the anti-estrogen ICI 182,780 to antagonize this effect, mitogenic effects of E2 on epithelial proliferation appear to be mediated through stromal ER.
Growth factors could be involved in mediating the mitogenic effects of E2 on UtE. A variety of growth factors are produced by stroma from uterus and other estrogen target organs, and in many cases, epithelial growth factor receptors and/or biological actions of growth factors on epithelial cells have been described. For example, E2 increases expression of epidermal growth factor and its receptor in uterus (24–26), and many in vivo effects of E2 can be elicited in UtE of ovariectomized mice by exogenous epidermal growth factor (27). Insulin-like growth factor-1 is produced in relatively high amounts in uterus, preferentially in stroma (28), and is a potent epithelial mitogen, which is regulated by E2 in uterus (28) and could function as a paracrine mediator of mitogenic effects of E2 on UtE. Hepatocyte growth factor is a mesenchymally derived heparin-binding cytokine. Its receptor, the product of the c-met proto-oncogene, is predominately found in epithelium. Hepatocyte growth factor stimulates epithelial mitogenesis in several organs (29, 30). Another growth factor that could be involved in mesenchymal-epithelial interactions is keratinocyte growth factor, which is secreted by mesenchymal and fibroblastic cells and stimulates epithelial cell proliferation (31). Keratinocyte growth factor is expressed in monkey uterus (32). In other estrogen target organs such as the mammary gland, keratinocyte growth factor stimulates epithelial proliferation in vitro (33) and in vivo (34, 35). Other growth factors such as transforming growth factor-α could also be involved in UtS+UtE interactions (36).
Lack of involvement of epithelial ER in estrogen-induced epithelial mitogenesis raises important questions as to the role of epithelial ER in uterus. E2 directly induces progesterone receptor in UtE in vitro (11), clearly indicating that some E2 effects on UtE normally seen in vivo may be mediated directly through epithelial ER. Again, previous work with androgens and male reproductive epithelia may be useful in distinguishing direct from indirect E2 responses in female reproductive epithelia. Although epithelial androgen receptors also are not necessary for androgen-induced epithelial mitogenesis, epithelial androgen receptors are essential for production of epithelial secretory proteins (37, 38). By analogy, epithelial ER may also be essential for estrogen-induced production of epithelial secretory proteins. Therefore, E2 action on UtE may reflect both direct and indirect stromally mediated effects; the use of tissue separation/recombination techniques in conjunction with ERKO mice should provide an effective experimental methodology to explore these questions in uterus, mammary gland, and other estrogen responsive organs.
ER-β, a novel member of the steroid receptor superfamily, was recently cloned (39). Homology of ER-β to the classical estrogen receptor is extensive in the DNA-binding domain, but less pronounced in other regions of the molecule. In vitro protein expression studies have shown that ER-β binds E2, has a molecular mass of 54 kDa and can activate transcription through an estrogen response element. ER-β mRNA has been detected in rat prostate and ovary by reverse transcription-PCR (39), and also can be detected in uterus of ERKO mice (Gustafsson et al., unpublished observations). However, E2 does not produce characteristic estrogenic responses in ERKO mice such as uterine epithelial DNA synthesis or increases in uterine wet weight and mRNA levels for uterine progesterone receptor, glucose-6-phosphate dehydrogenase or lactoferrin (40). Therefore, ER-β does not seem to be involved in proliferative effects of E2 on UtE, and mitogenic effects of E2 observed in wt-S + ko-E tissue recombinants would appear to be mediated by ER-α, not ER-β.
Recent work by Couse et al. (40) has shown that full-length wt-ER mRNA is undetectable in ERKO mice, but two smaller ER transcripts are present at extremely low levels. In these variants, designated E1 and E2, the disrupting neomycin resistance sequence was completely or partially spliced from the mRNA (40). Because the ER reading frame in E1 was preserved, the E1 fragment could theoretically encode a smaller ER variant that still possesses DNA and ligand binding domains and therefore may be responsible (in addition to ER-β) for residual E2 binding detected in uteri of ERKO mice (16). However, E2 does not induce typical estrogenic responses in ERKO uteri, as explained above. Therefore, though a splicing variant of the disrupted ER gene may exist in ERKO uteri, it is either nonfunctional or at levels below that required to mediate a physiological response to E2 (40), and could not mediate direct E2-induced epithelial mitogenesis in ko-E associated with wt-S.
Previous work has shown that androgen-induced epithelial proliferation in the prostate gland is mediated indirectly through mesenchymal/stromal androgen receptors (41). In addition, recent experiments using tissue from the progesterone receptor knockout mouse have indicated that the inhibitory effect of progesterone on estrogen-induced epithelial proliferation is mediated through stromal progesterone receptors (G.R.C. and P.Y., unpublished work). These indirect effects of androgens and progestins on epithelial proliferation, in combination with the estrogen data present here, suggest that epithelial growth regulation in male and female reproductive organs proceeds through common paracrine mechanisms mediated by stromal hormone receptors. Furthermore, additional work is needed to determine if this mode of action may be common to other steroid hormones and possibly even other hormones whose receptors are members of the steroid/thyroid hormone/retinoic acid receptor superfamily.
In conclusion, the present data provide evidence that mitogenic effects of E2 on UtE are mediated indirectly via stromal ER. These findings clearly have relevance for understanding how E2 normally affects growth and functional differentiation of the epithelium of uterus and other reproductive organs. In addition, a number of serious and common health problems, such as endometriosis and endometrial cancer, involve aberrant proliferation of UtE, and E2 is at least a permissive agent in these diseases. Therefore, an increased understanding of how E2 normally regulates UtE may contribute to the understanding of these pervasive female reproductive health problems as well.
Acknowledgments
This work was supported by National Institutes of Health Grant HD 29376 (P.S.C.), CA 05388, and AG 13784 (G.R.C.) and a grant from the Molecular Biology Program of the University of Missouri (D.B.L.).
ABBREVIATIONS
- ER
estrogen receptor
- ERKO
estrogen receptor knockout
- S
stroma
- E
epithelium
- E2
estradiol-17β
- ARG
autoradiography
- UtE
uterine epithelium
- UtS
uterine stroma
- wt
wild type
- ko
knockout
References
- 1.Bigsby R M, Aixin L, Luo K, Cunha G R. Endocrinology. 1990;126:2592–2596. doi: 10.1210/endo-126-5-2592. [DOI] [PubMed] [Google Scholar]
- 2.Clark J H, Peck E J. Female Sex Steroids: Receptors and Function. New York: Springer; 1979. [PubMed] [Google Scholar]
- 3.Medlock K L, Sheehan D M, Branham W S. J Steroid Biochem. 1981;15:283–288. doi: 10.1016/0022-4731(81)90285-5. [DOI] [PubMed] [Google Scholar]
- 4.Stumpf W, Sar M. In: Receptors and Mechanism of Action of Steroid Hormones. Pasqualini J, editor. New York: Dekker; 1976. pp. 41–84. [Google Scholar]
- 5.Cunha G R, Shannon J M, Vanderslice K D, Sekkingstad M, Robboy S J. J Steroid Biochem. 1982;17:281–286. doi: 10.1016/0022-4731(82)90201-1. [DOI] [PubMed] [Google Scholar]
- 6.Greco T L, Duello T M, Gorski J. Endocr Rev. 1993;14:59–71. doi: 10.1210/edrv-14-1-59. [DOI] [PubMed] [Google Scholar]
- 7.Li S. Histochemistry. 1994;102:405–413. doi: 10.1007/BF00268912. [DOI] [PubMed] [Google Scholar]
- 8.Bigsby R M, Cunha G R. Endocrinology. 1986;119:390–396. doi: 10.1210/endo-119-1-390. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita S, Newbold R R, McLachlan J A, Korach K S. Endocrinology. 1990;127:2456–2463. doi: 10.1210/endo-127-5-2456. [DOI] [PubMed] [Google Scholar]
- 10.Casimiri V, Tath N C, Parvey H, Psychoyos A. J Steroid Biochem. 1980;12:293–298. doi: 10.1016/0022-4731(80)90282-4. [DOI] [PubMed] [Google Scholar]
- 11.Uchima F-D A, Edery M, Iguchi T, Bern H A. J Endocrinol. 1991;128:115–120. doi: 10.1677/joe.0.1280115. [DOI] [PubMed] [Google Scholar]
- 12.Julian J, Carson D D, Glasser S R. Endocrinology. 1992;130:79–87. doi: 10.1210/endo.130.1.1727726. [DOI] [PubMed] [Google Scholar]
- 13.Cooke P S, Uchima F-D A, Fujii D K, Bern H A, Cunha G R. Proc Natl Acad Sci USA. 1986;83:2109–2113. doi: 10.1073/pnas.83.7.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inaba T, Wiest W G, Strickler R C, Mori J. Endocrinology. 1988;123:1253–1258. doi: 10.1210/endo-123-3-1253. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita S, Newbold R R, McLachlan J A, Korach K S. Endocrinology. 1989;125:2888–2896. doi: 10.1210/endo-125-6-2888. [DOI] [PubMed] [Google Scholar]
- 16.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korach K S. Science. 1994;266:1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- 18.Korach K S, Couse J F, Curtis S W, Washburn T F, Lindzey J, Kimbro K S, Lubahn D B, Eddy E M, Snedeker S M, Schomberg D W, Smith E P. Rec Prog Horm Res. 1996;51:159–188. [PubMed] [Google Scholar]
- 19.Bigsby R M, Cooke P S, Cunha G R. Am J Physiol. 1986;251:E630–E636. doi: 10.1152/ajpendo.1986.251.5.E630. [DOI] [PubMed] [Google Scholar]
- 20.Cunha G R. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- 21.Cunha G R, Young P. Epithelial Cell Biol. 1992;1:18–31. [PubMed] [Google Scholar]
- 22.Slayden O D, Koji T, Brenner R M. Endocrinology. 1995;136:4012–4021. doi: 10.1210/endo.136.9.7649110. [DOI] [PubMed] [Google Scholar]
- 23.Langdon S P, Crew A J, Ritchie A A, Muir M, Wakeling A, Smyth J F, Miller W R. Eur J Cancer. 1994;30:682–686. doi: 10.1016/0959-8049(94)90545-2. [DOI] [PubMed] [Google Scholar]
- 24.Mukku V R, Stancel G M. Endocrinology. 1985;117:149–154. doi: 10.1210/endo-117-1-149. [DOI] [PubMed] [Google Scholar]
- 25.Mukku V R, Stancel G M. J Biol Chem. 1985;260:9820–9824. [PubMed] [Google Scholar]
- 26.Di Augustine R P, Petrusz P, Bell G I, Brown C F, Korach K S, McLachlan J A, Teng C T. Endocrinology. 1988;122:2355–2363. doi: 10.1210/endo-122-6-2355. [DOI] [PubMed] [Google Scholar]
- 27.Nelson K G, Takahashi T, Bossert N L, Walmer D K, McLachlan J A. Proc Natl Acad Sci USA. 1991;88:21–25. doi: 10.1073/pnas.88.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy L J, Ghahary A. Endocr Rev. 1990;11:443–453. doi: 10.1210/edrv-11-3-443. [DOI] [PubMed] [Google Scholar]
- 29.Zarnegar R, Michalopoulos G. J Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen E R, Nigam S K, Goldberg I D. J Cell Biol. 1995;127:1783–1787. doi: 10.1083/jcb.127.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aaronson S A, Rubin J S, Finch P W, Wong J, Marchese C, Falco J, Taylor W G, Kraus M H. Am Rev Respir Dis. 1990;142:S7–S10. doi: 10.1164/ajrccm/142.6_Pt_2.S7. [DOI] [PubMed] [Google Scholar]
- 32.Koji T, Chedid M, Rubin J S, Slayden O D, Csaky K G, Aaronson S A, Brenner R M. J Cell Biol. 1994;125:393–401. doi: 10.1083/jcb.125.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imagawa W, Cunha G R, Young P, Nandi S. Biochem Biophys Res Commun. 1994;204:1165–1169. doi: 10.1006/bbrc.1994.2585. [DOI] [PubMed] [Google Scholar]
- 34.Ulich T R, Yi E S, Cardiff R, Yin S, Bikhazi N, Biltz R, Morris C F, Pierce G F. Am J Pathol. 1994;144:862–868. [PMC free article] [PubMed] [Google Scholar]
- 35.Yi E S, Bedoya A A, Lee H, Kim S, Housley R M, Aukerman S L, Tarpley J E, Starnes C, Yin S, Pierce G F. Am J Pathol. 1994;145:1015–1022. [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson K G, Takahashi T, Lee D C, Luetteke N C, Bossert N L, Ross K, Eitzman B E, McLachlan J A. Endocrinology. 1992;131:1657–1664. doi: 10.1210/endo.131.4.1396310. [DOI] [PubMed] [Google Scholar]
- 37.Cunha G R, Young P. Endocrinology. 1991;128:3293–3298. doi: 10.1210/endo-128-6-3293. [DOI] [PubMed] [Google Scholar]
- 38.Donjacour A A, Cunha G R. Endocrinology. 1993;131:2342–2350. doi: 10.1210/endo.132.6.7684975. [DOI] [PubMed] [Google Scholar]
- 39.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-A. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couse J F, Curtis S W, Washburn T F, Lindzey J, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 41.Cunha G R, Alarid E T, Turner T, Donjacour A A, Boutin E L, Foster B A. J Androl. 1992;13:465–475. [PubMed] [Google Scholar]