Abstract
The lambda S lysis gene was cloned into a Saccharomyces cerevisiae expression vector under GAL1 control. Induction with galactose in S. cerevisiae terminated cell growth and prevented colony formation. Several membrane proteins immunoreactive with anti-S antibody accumulated in the membranes, indicating that sodium dodecyl sulfate-resistant oligomers of S are formed, similar to those observed in the membranes of Escherichia coli cells killed by expression of the S gene. These observations suggest that the S gene product functions as a cytotoxic protein in the yeast cytoplasmic membrane as it does in the bacterial membrane.
Full text
PDF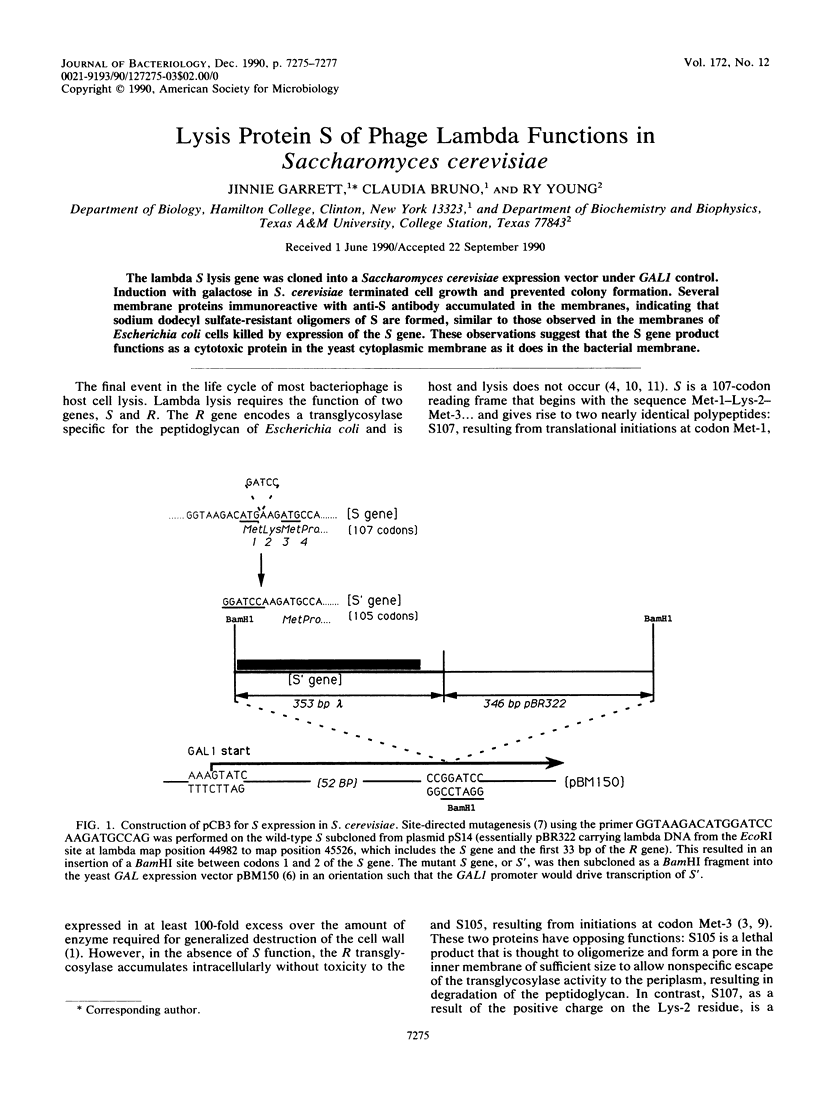


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowska-Szewczyk K., Lipinska B., Taylor A. The R gene product of bacteriophage lambda is the murein transglycosylase. Mol Gen Genet. 1981;184(1):111–114. doi: 10.1007/BF00271205. [DOI] [PubMed] [Google Scholar]
- Bläsi U., Chang C. Y., Zagotta M. T., Nam K. B., Young R. The lethal lambda S gene encodes its own inhibitor. EMBO J. 1990 Apr;9(4):981–989. doi: 10.1002/j.1460-2075.1990.tb08200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsi U., Nam K., Hartz D., Gold L., Young R. Dual translational initiation sites control function of the lambda S gene. EMBO J. 1989 Nov;8(11):3501–3510. doi: 10.1002/j.1460-2075.1989.tb08515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. M., Young R. Lethal action of bacteriophage lambda S gene. J Virol. 1982 Dec;44(3):886–892. doi: 10.1128/jvi.44.3.886-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Nam K., Bläsi U., Zagotta M. T., Young R. Conservation of a dual-start motif in P22 lysis gene regulation. J Bacteriol. 1990 Jan;172(1):204–211. doi: 10.1128/jb.172.1.204-211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab R., Neal G., Sohaskey C., Smith J., Young R. Dominance in lambda S mutations and evidence for translational control. J Mol Biol. 1988 Jan 5;199(1):95–105. doi: 10.1016/0022-2836(88)90381-6. [DOI] [PubMed] [Google Scholar]
- Reader R. W., Siminovitch L. Lysis defective mutants of bacteriophage lambda: genetics and physiology of S cistron mutants. Virology. 1971 Mar;43(3):607–622. doi: 10.1016/0042-6822(71)90286-8. [DOI] [PubMed] [Google Scholar]
- Rolfe B. G., Campbell J. H. A relationship between tolerance to colicin K and the mechanism of phage-induced host cell lysis. Mol Gen Genet. 1974;133(4):293–297. doi: 10.1007/BF00332705. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta M. T., Wilson D. B. Oligomerization of the bacteriophage lambda S protein in the inner membrane of Escherichia coli. J Bacteriol. 1990 Feb;172(2):912–921. doi: 10.1128/jb.172.2.912-921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]




