Abstract
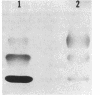
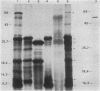
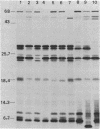
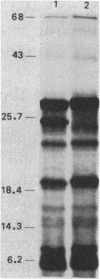
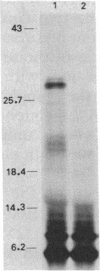
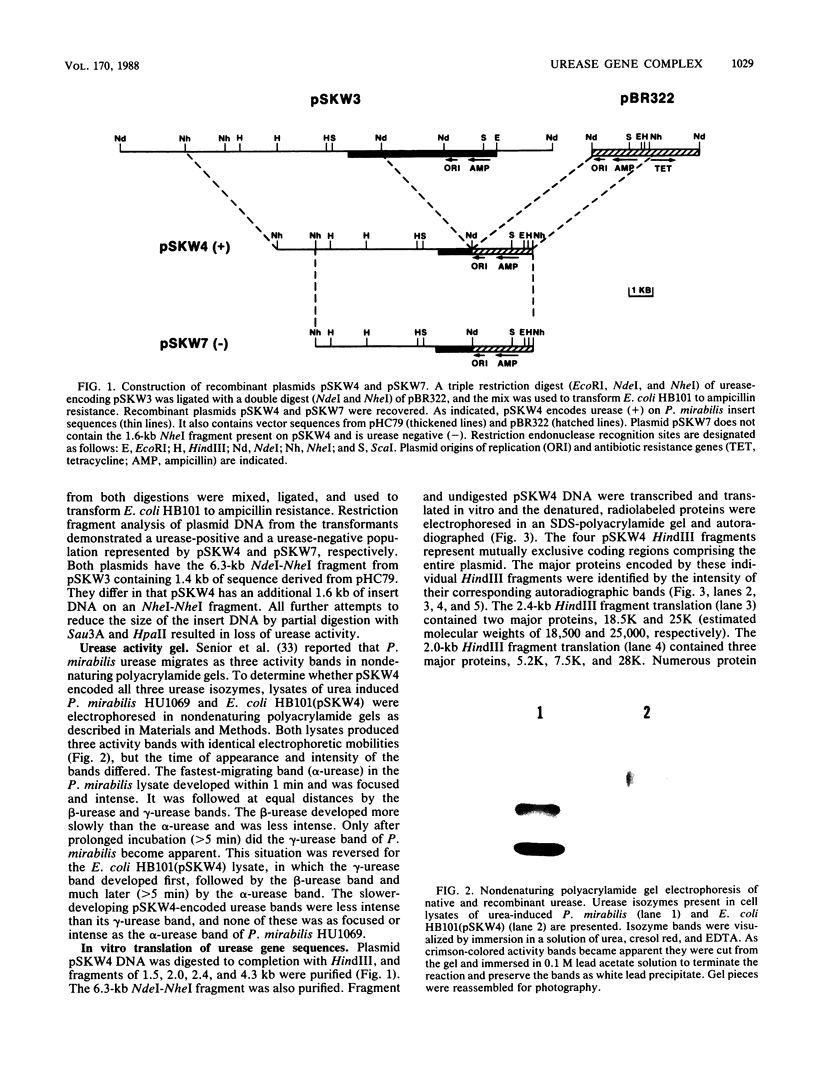
Chromosomal DNA fragments from a uropathogenic isolate of Proteus mirabilis were inserted into the cosmid vector pHC79 to construct a genomic library in Escherichia coli HB101. A urease-positive recombinant cosmid, designated pSKW1, was recovered. Sequential recombinant manipulation of pSKW1 yielded a 10.2-kilobase plasmid, designated pSKW4, which encoded three urease isozymes with electrophoretic mobilities identical to those of the donor P. mirabilis strain. Plasmid pSKW4 gene sequences encode seven proteins designated 68K (apparent molecular weight, of 68,000), 28K, 25K, 22.5K, 18.5K, 7.5K, and 5.2K within the limits of the urease gene complex. Insertion mutations in genes encoding the 68K, 28K, 25K, 22.5K, 7.5K, and 5.2K proteins resulted in complete or partial (22.5K) loss of urease activity. There was no reduction in urease activity when the gene encoding the 18.5K protein was inactivated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUDE A. I., SIEMIENSKI J. Role of bacterial urease in experimental pyelonephritis. J Bacteriol. 1960 Aug;80:171–179. doi: 10.1128/jb.80.2.171-179.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattler D. P., Contaxis C. C., Reithel F. J. Dissociation of urease by glycol and glycerol. Nature. 1967 Oct 21;216(5112):274–275. doi: 10.1038/216274b0. [DOI] [PubMed] [Google Scholar]
- Christensen W. B. Urea Decomposition as a Means of Differentiating Proteus and Paracolon Cultures from Each Other and from Salmonella and Shigella Types. J Bacteriol. 1946 Oct;52(4):461–466. doi: 10.1128/jb.52.4.461-466.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians S., Kaltwasser H. Nickel-content of urease from Bacillus pasteurii. Arch Microbiol. 1986 Jun;145(1):51–55. doi: 10.1007/BF00413026. [DOI] [PubMed] [Google Scholar]
- Cicmanec J. F., Helmers S. L., Evans A. T. Office practice survey of urease positive bacterial pathogens causing urinary tract infections. Urology. 1980 Sep;16(3):274–276. doi: 10.1016/0090-4295(80)90041-2. [DOI] [PubMed] [Google Scholar]
- Dixon N. E., Hinds J. A., Fihelly A. K., Gazzola C., Winzor D. J., Blakeley R. L., Zerner B. Jack bean urease (EC 3.5.1.5). IV. The molecular size and the mechanism of inhibition by hydroxamic acids. Spectrophotometric titration of enzymes with reversible inhibitors. Can J Biochem. 1980 Dec;58(12):1323–1334. doi: 10.1139/o80-180. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Urease of Klebsiella aerogenes: control of its synthesis by glutamine synthetase. J Bacteriol. 1977 Aug;131(2):446–452. doi: 10.1128/jb.131.2.446-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO M. M., LIU P. V. SEROLOGICAL SPECIFICITIES OF UREASES OF PROTEUS SPECIES. J Gen Microbiol. 1965 Mar;38:417–422. doi: 10.1099/00221287-38-3-417. [DOI] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Hase J., Kobashi K. Inhibition of Proteus vulgaris urease by hydroxamic acids. J Biochem. 1967 Sep;62(3):293–299. [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R., Bieler S., Falkow S., Hull S. Chromosomal map position of genes encoding P adhesins in uropathogenic Escherichia coli. Infect Immun. 1986 Feb;51(2):693–695. doi: 10.1128/iai.51.2.693-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Genetic and biochemical diversity of ureases of Proteus, Providencia, and Morganella species isolated from urinary tract infection. Infect Immun. 1987 Sep;55(9):2198–2203. doi: 10.1128/iai.55.9.2198-2203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBASHI K., HASE J., UEHARA K. Specific inhibition of urease by hydroxamic acids. Biochim Biophys Acta. 1962 Dec 4;65:380–383. doi: 10.1016/0006-3002(62)91067-3. [DOI] [PubMed] [Google Scholar]
- LEAL J. A., VILLANUEVA J. R. An improved selective medium for the formation of ascospores by Aspergillus nidulans. Nature. 1962 Mar 17;193:1106–1106. doi: 10.1038/1931106a0. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacLaren D. M. The significance of urease in proteus pyelonephritis: a histological and biochemical study. J Pathol. 1969 Jan;97(1):43–49. doi: 10.1002/path.1710970107. [DOI] [PubMed] [Google Scholar]
- Magaña-Plaza I., Ruiz-Herrera J. Mechanisms of regulation of urease biosynthesis in Proteus rettgeri. J Bacteriol. 1967 Apr;93(4):1294–1301. doi: 10.1128/jb.93.4.1294-1301.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. J., Cheng K. J., Gould W. D., Nickel J. C., Costerton J. W. Histochemical and biochemical urease localization in the periplasm and outer membrane of two Proteus mirabilis strains. Can J Microbiol. 1986 Oct;32(10):772–778. doi: 10.1139/m86-142. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Jones B. D., Jerse A. E. Cloning of urease gene sequences from Providencia stuartii. Infect Immun. 1986 Oct;54(1):161–169. doi: 10.1128/iai.54.1.161-169.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J. M., Boulnois G. J., Darby V., Orr E., Wahle E., Holland I. B. Identification of gene products programmed by restriction endonuclease DNA fragments using an E. coli in vitro system. Nucleic Acids Res. 1981 Sep 25;9(18):4459–4474. doi: 10.1093/nar/9.18.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Hamilton-Miller J. M., Brumfitt W. Role of urease in the formation of infection stones: comparison of ureases from different sources. Infect Immun. 1981 Apr;32(1):32–37. doi: 10.1128/iai.32.1.32-37.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Hamilton-Miller J. M. Inhibitors of urease as chemotherapeutic agents. Crit Rev Microbiol. 1984;11(1):1–12. doi: 10.3109/10408418409105901. [DOI] [PubMed] [Google Scholar]
- Rosenstein I., Hamilton-Miller J. M., Brumfitt W. The effect of acetohydroxamic acid on the induction of bacterial ureases. Invest Urol. 1980 Sep;18(2):112–114. [PubMed] [Google Scholar]
- Senior B. W., Bradford N. C., Simpson D. S. The ureases of Proteus strains in relation to virulence for the urinary tract. J Med Microbiol. 1980 Nov;13(4):507–512. doi: 10.1099/00222615-13-4-507. [DOI] [PubMed] [Google Scholar]
- Shaik-M M. B., Guy A. L., Pancholy S. K. An improved method for the detection and preservation of urease activity in polyacrylamide gels. Anal Biochem. 1980 Mar 15;103(1):140–143. doi: 10.1016/0003-2697(80)90247-x. [DOI] [PubMed] [Google Scholar]
- So M., Dallas W. S., Falkow S. Characterization of an Escherichia coli plasmid encoding for synthesis of heat-labile toxin: molecular cloning of the toxin determinant. Infect Immun. 1978 Aug;21(2):405–411. doi: 10.1128/iai.21.2.405-411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart C. A., Van Stratum E., Rustigian R. Further Studies on Urease Production by Proteus and Related Organisms. J Bacteriol. 1945 May;49(5):437–444. doi: 10.1128/jb.49.5.437-444.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuye A., Pijck J. Urease activity of enterobacteriaceae: which medium to choose. Appl Microbiol. 1973 Dec;26(6):850–854. doi: 10.1128/am.26.6.850-854.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartell R. M., Reznikoff W. S. Cloning DNA restriction endonuclease fragments with protruding single-stranded ends. Gene. 1980 May;9(3-4):307–319. doi: 10.1016/0378-1119(90)90329-p. [DOI] [PubMed] [Google Scholar]
- Wray S. K., Hull S. I., Cook R. G., Barrish J., Hull R. A. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect Immun. 1986 Oct;54(1):43–49. doi: 10.1128/iai.54.1.43-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn C., Dietrich R., Kaltwasser H. Regulation by repression of urease biosynthesis in Proteus rettgeri. Z Allg Mikrobiol. 1982;22(3):197–203. doi: 10.1002/jobm.3630220308. [DOI] [PubMed] [Google Scholar]