Abstract
Mutant phoR cells show a clonal variation phenotype with respect to bacterial alkaline phosphatase (BAP) synthesis. BAP clonal variation is characterized by an alternation between a Bap+ and Bap- phenotype. The switching is regulated by the phoM operon and the presence of glucose; the pho-510 mutant form of the phoM operon abolishes both BAP clonal variation and the effect of glucose (B.L. Wanner, J. Bacteriol. 169:900-903, 1987). In this paper we show that a mutation of the adenyl cyclase (cya) and the cyclic AMP receptor protein (crp) gene also abolish BAP clonal variation; either simultaneously reduces the amount of BAP made in phoR mutants. Also, the pho-510 mutation is epistatic; it increases BAP synthesis in delta cya phoR and delta crp phoR mutants. These data are consistent with the wild-type phoM operon having a negative, as well as a positive, regulatory role in gene expression. Furthermore, the data suggest that adenyl cyclase and Crp indirectly regulate BAP synthesis in a phoR mutant via an interaction with the phoM operon or its gene products. However, phoM operon expression was unaffected when tested with phoM operon lacZ transcriptional fusions. In addition, the switching Bap phenotype was not associated with an alternation in phoM operon expression.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemura M., Makino K., Shinagawa H., Nakata A. Nucleotide sequence of the phoM region of Escherichia coli: four open reading frames may constitute an operon. J Bacteriol. 1986 Oct;168(1):294–302. doi: 10.1128/jb.168.1.294-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S., Drury L. S. Cloning and insertional inactivation of the dye (sfrA) gene, mutation of which affects sex factor F expression and dye sensitivity of Escherichia coli K-12. J Bacteriol. 1983 Jun;154(3):1309–1314. doi: 10.1128/jb.154.3.1309-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury L. S., Buxton R. S. DNA sequence analysis of the dye gene of Escherichia coli reveals amino acid homology between the dye and OmpR proteins. J Biol Chem. 1985 Apr 10;260(7):4236–4242. [PubMed] [Google Scholar]
- Fassler J. S., Tessman I., Tessman E. S. Lethality of the double mutations rho rep and rho ssb in Escherichia coli. J Bacteriol. 1985 Feb;161(2):609–614. doi: 10.1128/jb.161.2.609-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Directed formation of deletions and duplications using Mud(Ap, lac). Genetics. 1985 Feb;109(2):263–282. doi: 10.1093/genetics/109.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil D. General method, using Mu-Mud1 dilysogens, to determine the direction of transcription of and generate deletions in the glnA region of Escherichia coli. J Bacteriol. 1981 Apr;146(1):260–268. doi: 10.1128/jb.146.1.260-268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Nakata A. Regulation of the phosphate regulon of Escherichia coli K-12: regulation and role of the regulatory gene phoR. J Mol Biol. 1985 Jul 20;184(2):231–240. doi: 10.1016/0022-2836(85)90376-6. [DOI] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Simon M., Zieg J., Silverman M., Mandel G., Doolittle R. Phase variation: evolution of a controlling element. Science. 1980 Sep 19;209(4463):1370–1374. doi: 10.1126/science.6251543. [DOI] [PubMed] [Google Scholar]
- Wanner B. L. Bacterial alkaline phosphatase clonal variation in some Escherichia coli K-12 phoR mutant strains. J Bacteriol. 1986 Dec;168(3):1366–1371. doi: 10.1128/jb.168.3.1366-1371.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Bernstein J. Determining the phoM map location in Escherichia coli K-12 by using a nearby transposon Tn10 insertion. J Bacteriol. 1982 Apr;150(1):429–432. doi: 10.1128/jb.150.1.429-432.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Chang B. D. The phoBR operon in Escherichia coli K-12. J Bacteriol. 1987 Dec;169(12):5569–5574. doi: 10.1128/jb.169.12.5569-5574.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
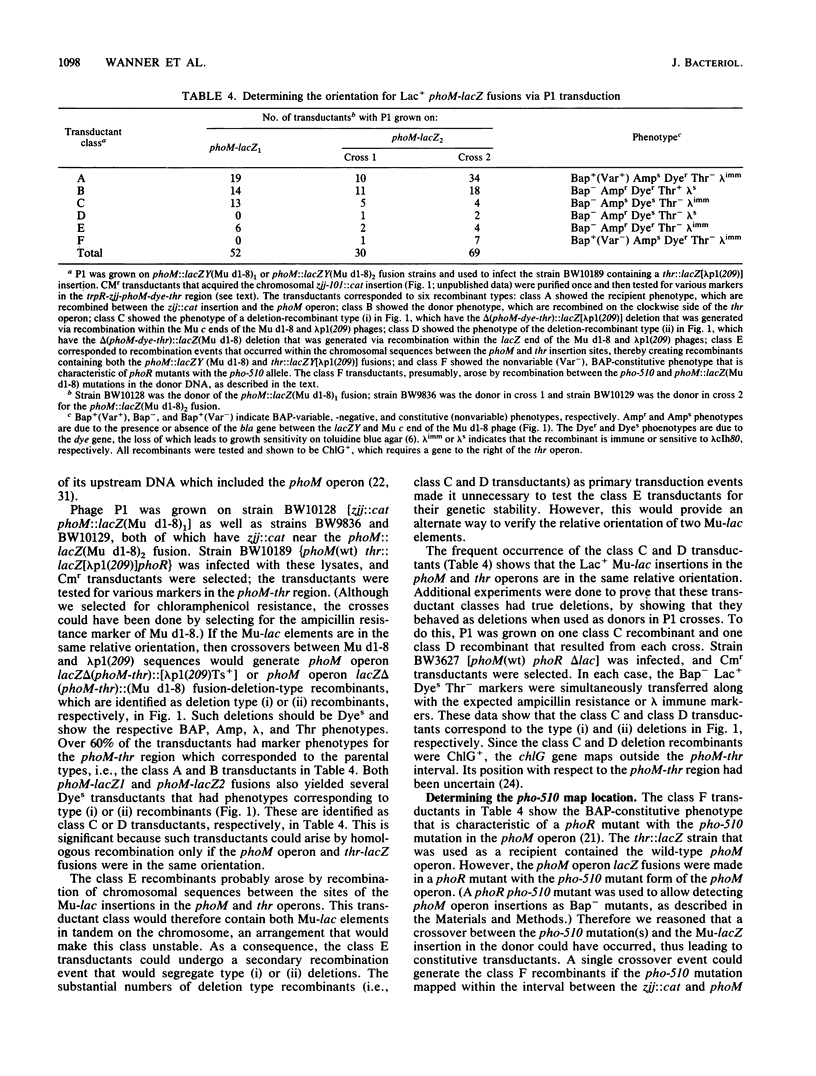
- Wanner B. L. Control of phoR-dependent bacterial alkaline phosphatase clonal variation by the phoM region. J Bacteriol. 1987 Feb;169(2):900–903. doi: 10.1128/jb.169.2.900-903.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Regulation of lac operon expression: reappraisal of the theory of catabolite repression. J Bacteriol. 1978 Dec;136(3):947–954. doi: 10.1128/jb.136.3.947-954.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Latterell P. Mutants affected in alkaline phosphatase, expression: evidence for multiple positive regulators of the phosphate regulon in Escherichia coli. Genetics. 1980 Oct;96(2):353–366. doi: 10.1093/genetics/96.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., McSharry R. Phosphate-controlled gene expression in Escherichia coli K12 using Mudl-directed lacZ fusions. J Mol Biol. 1982 Jul 5;158(3):347–363. doi: 10.1016/0022-2836(82)90202-9. [DOI] [PubMed] [Google Scholar]
- Wanner B. L. Molecular cloning of Mu d(bla lacZ) transcriptional and translational fusions. J Bacteriol. 1987 May;169(5):2026–2030. doi: 10.1128/jb.169.5.2026-2030.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J Mol Biol. 1986 Sep 5;191(1):39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Sarthy A., Beckwith J. Escherichia coli pleiotropic mutant that reduces amounts of several periplasmic and outer membrane proteins. J Bacteriol. 1979 Oct;140(1):229–239. doi: 10.1128/jb.140.1.229-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Wieder S., McSharry R. Use of bacteriophage transposon Mu d1 to determine the orientation for three proC-linked phosphate-starvation-inducible (psi) genes in Escherichia coli K-12. J Bacteriol. 1981 Apr;146(1):93–101. doi: 10.1128/jb.146.1.93-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Wilmes M. R., Hunter E. Molecular cloning of the wild-type phoM operon in Escherichia coli K-12. J Bacteriol. 1988 Jan;170(1):279–288. doi: 10.1128/jb.170.1.279-288.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
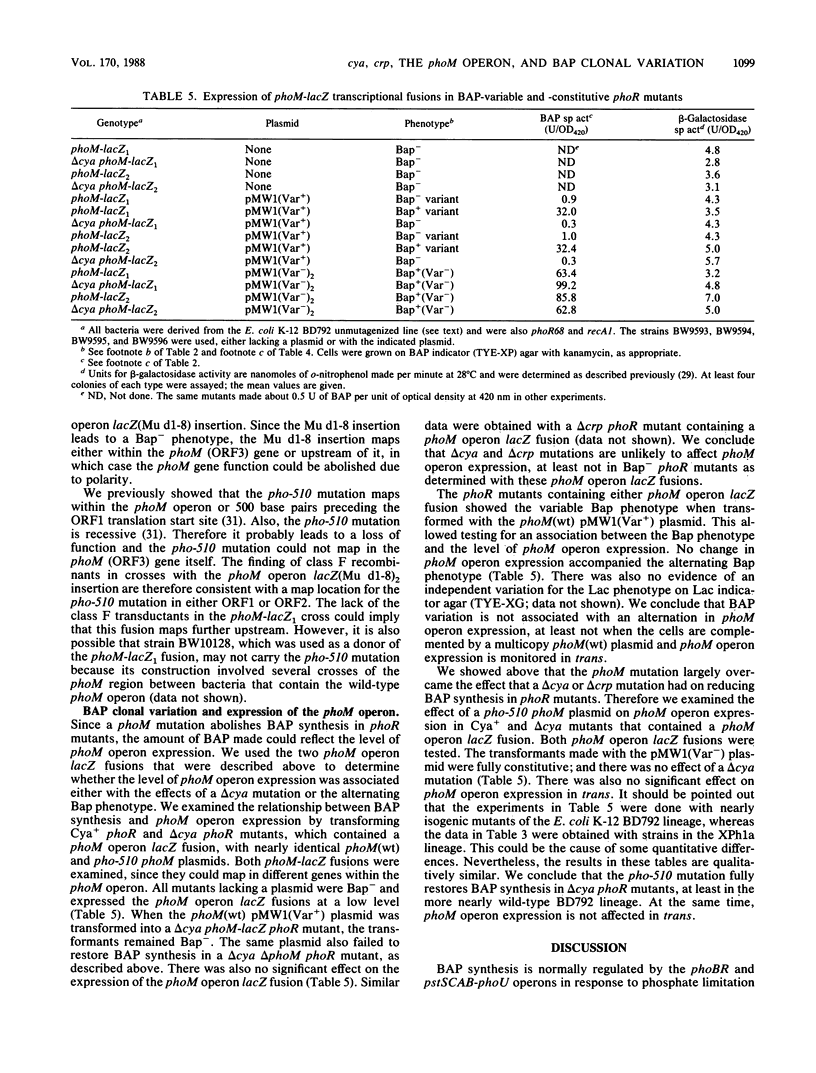
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]



