Abstract
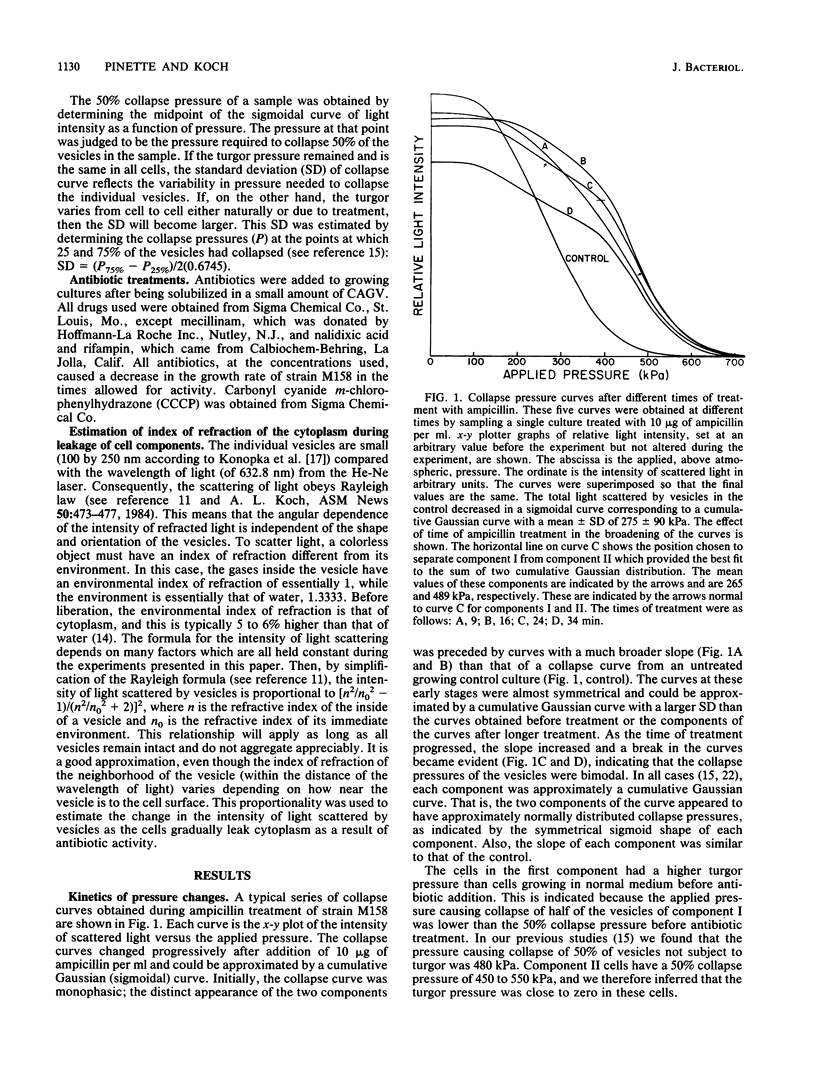
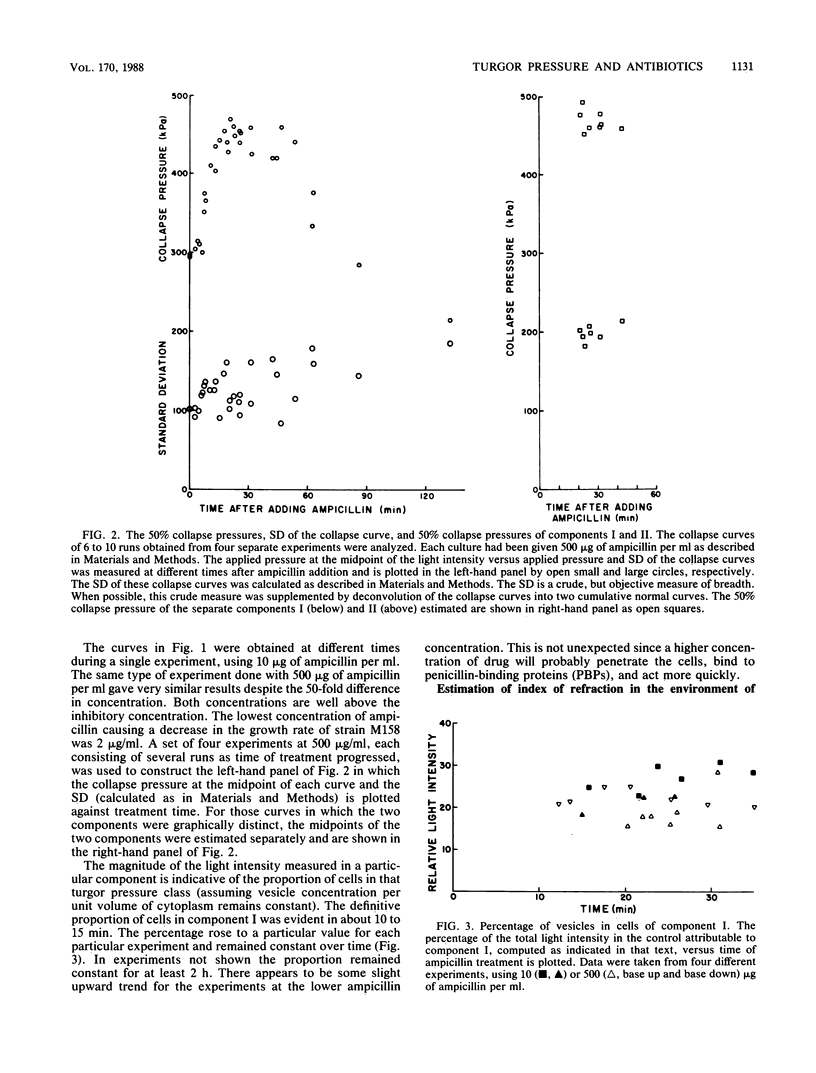
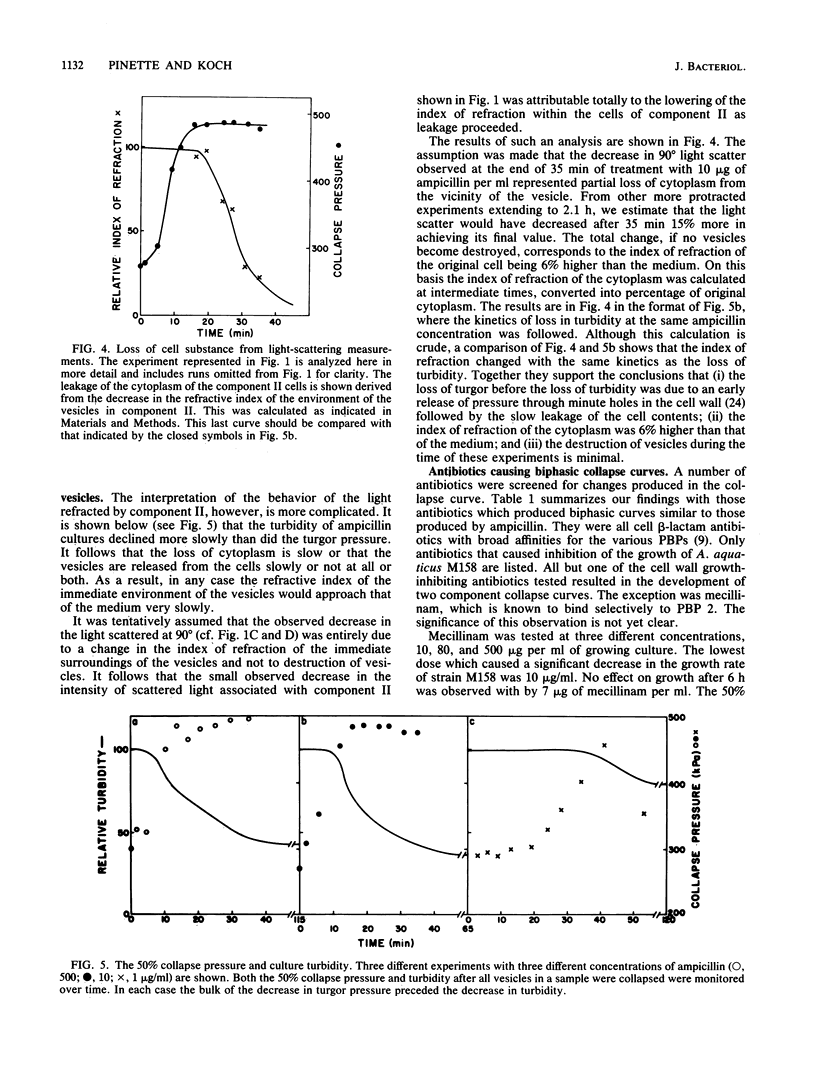
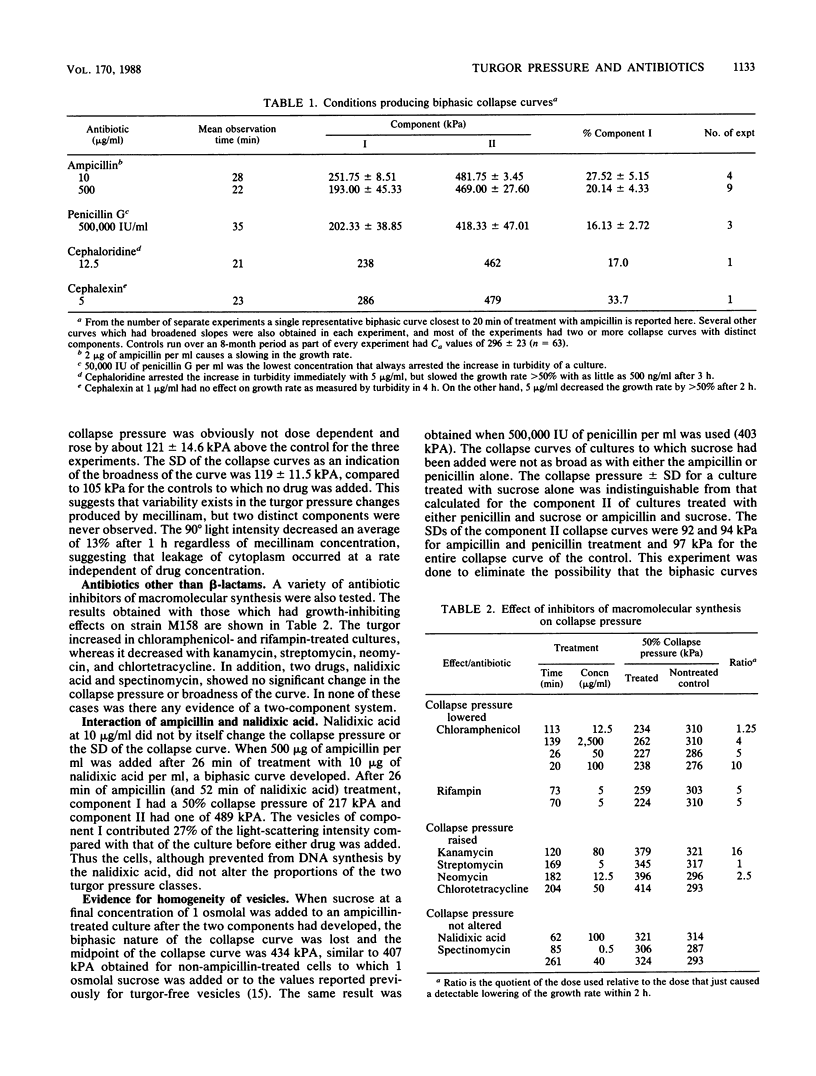
The internal hydrostatic pressure of Ancylobacter aquaticus was measured by collapsing the gas vesicles with an externally applied pressure. Turgor pressure was measured in conjunction with various antibiotic treatments to elucidate some aspects of the biophysics of gram-negative cell wall function. Differences in the effects of these drugs either alone or in combination with other treatments were related to known biochemical activities of these drugs. Our previous work, demonstrating a heterogeneous cellular response to beta-lactam antibodies, was confirmed and extended. Most of the cell wall growth-inhibiting antibiotics resulted in some cells (those in component I) developing a higher pressure, while the remainder (those in component II) lost turgor. Although the fraction of the cells in each component varied a little from subculture to subculture, it did not vary with time or choice of antibiotic treatment. Mecillinam gave a nearly monophasic response. All antibiotics blocking macromolecular synthesis gave monophasic curves. The 50% collapse pressure in some cases, however, was lower higher, or the same as the control.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Competition of beta-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrob Agents Chemother. 1979 Sep;16(3):325–328. doi: 10.1128/aac.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht P., Labischinski H., Wecke J. A special morphogenetic wall defect and the subsequent activity of "murosomes" as the very reason for penicillin-induced bacteriolysis in staphylococci. Arch Microbiol. 1985 May;141(4):315–324. doi: 10.1007/BF00428843. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Tomasz A. Antibiotic tolerance among clinical isolates of bacteria. Rev Infect Dis. 1985 May-Jun;7(3):368–386. doi: 10.1093/clinids/7.3.368. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Ferrero M., Daneo-Moore L. Relationship of shape to initiation of new sites of envelope growth in Streptococcus faecium cells treated with beta-lactam antibiotics. J Bacteriol. 1986 Aug;167(2):562–569. doi: 10.1128/jb.167.2.562-569.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A. L. Some calculations on the turbidity of mitochondria and bacteria. Biochim Biophys Acta. 1961 Aug 19;51:429–441. doi: 10.1016/0006-3002(61)90599-6. [DOI] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Escherichia coli mutants tolerant to beta-lactam antibiotics. J Bacteriol. 1979 Dec;140(3):955–963. doi: 10.1128/jb.140.3.955-963.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Burdett I. D. The variable T model for gram-negative morphology. J Gen Microbiol. 1984 Sep;130(9):2325–2338. doi: 10.1099/00221287-130-9-2325. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Ehrenfeld E. Th size and shape of bacteria by light scattering measurements. Biochim Biophys Acta. 1968 Sep 3;165(2):262–273. doi: 10.1016/0304-4165(68)90054-8. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Pinette M. F. Nephelometric determination of turgor pressure in growing gram-negative bacteria. J Bacteriol. 1987 Aug;169(8):3654–3663. doi: 10.1128/jb.169.8.3654-3663.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka A. E., Lara J. C., Staley J. T. Isolation and characterization of gas vesicles from Microcyclus aquaticus. Arch Microbiol. 1977 Mar 1;112(2):133–140. doi: 10.1007/BF00429325. [DOI] [PubMed] [Google Scholar]
- Konopka A. E., Staley J. T., Lara J. C. Gas vesicle assembly in Microcyclus aquaticus. J Bacteriol. 1975 Jun;122(3):1301–1309. doi: 10.1128/jb.122.3.1301-1309.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M., Kasra R., van Heijenoort J. Induction and control of the autolytic system of Escherichia coli. J Bacteriol. 1982 Oct;152(1):26–34. doi: 10.1128/jb.152.1.26-34.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychajlonka M., McDowell T. D., Shockman G. D. Inhibition of peptidoglycan, ribonucleic acid, and protein synthesis in tolerant strains of Streptococcus mutans. Antimicrob Agents Chemother. 1980 Apr;17(4):572–582. doi: 10.1128/aac.17.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinette M. F., Koch A. L. Variability of the turgor pressure of individual cells of the gram-negative heterotroph Ancylobacter aquaticus. J Bacteriol. 1987 Oct;169(10):4737–4742. doi: 10.1128/jb.169.10.4737-4742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinicke B., Blümel P., Labischinski H., Giesbrecht P. Neither an enhancement of autolytic wall degradation nor an inhibition of the incorporation of cell wall material are pre-requisites for penicillin-induced bacteriolysis in staphylococci. Arch Microbiol. 1985 May;141(4):309–314. doi: 10.1007/BF00428842. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F., Burdett I. D. The bactericidal action of beta-lactam antibiotics on an autolysin-deficient strain of Bacillus subtilis. J Gen Microbiol. 1983 Feb;129(2):465–478. doi: 10.1099/00221287-129-2-465. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D. AMINO-ACID DEPRIVATION AND BACTERIAL CELL-WALL SYNTHESIS. Trans N Y Acad Sci. 1963 Dec;26:182–195. doi: 10.1111/j.2164-0947.1963.tb01241.x. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Seufert W., Messer W. DnaA protein binding to the plasmid origin region can substitute for primosome assembly during replication of pBR322 in vitro. Cell. 1987 Jan 16;48(1):73–78. doi: 10.1016/0092-8674(87)90357-6. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Tuomanen E. Newly made enzymes determine ongoing cell wall synthesis and the antibacterial effects of cell wall synthesis inhibitors. J Bacteriol. 1986 Aug;167(2):535–543. doi: 10.1128/jb.167.2.535-543.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Yourassowsky E., Van der Linden M. P., Lismont M. J., Crokaert F., Glupczynski Y. Correlation between growth curve and killing curve of Escherichia coli after a brief exposure to suprainhibitory concentrations of ampicillin and piperacillin. Antimicrob Agents Chemother. 1985 Dec;28(6):756–760. doi: 10.1128/aac.28.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]



