Abstract
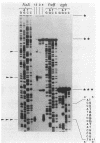
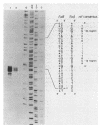
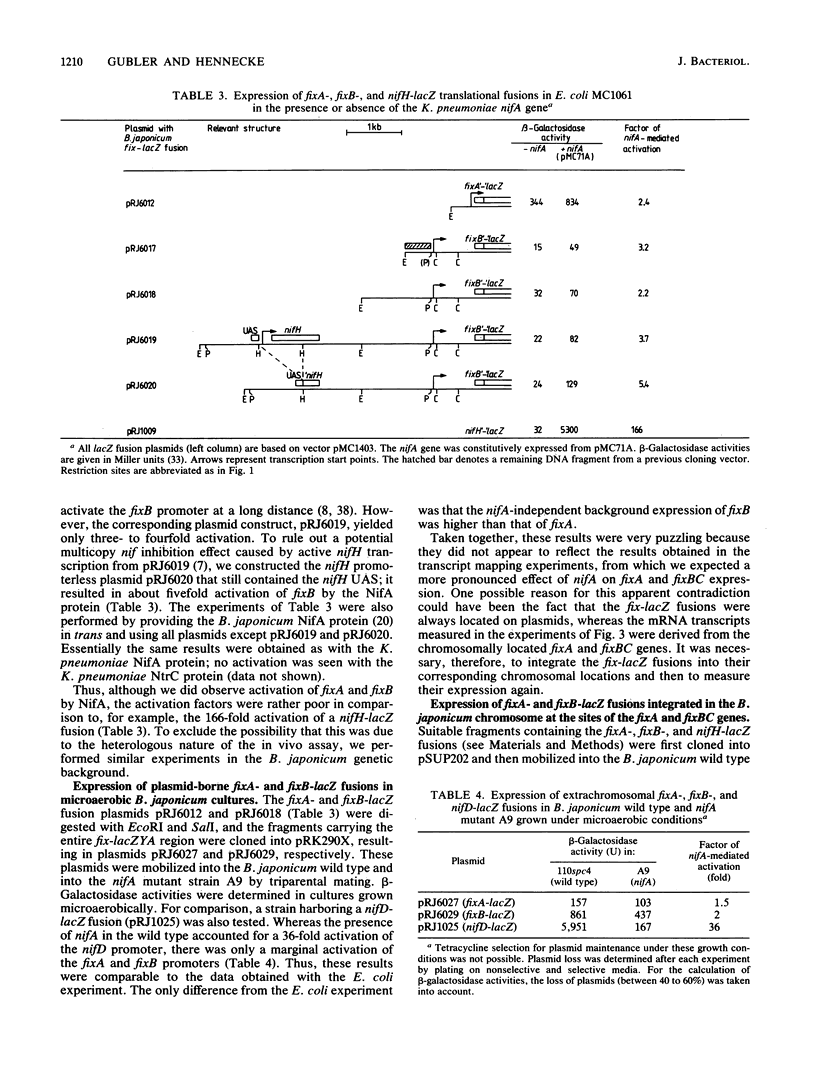
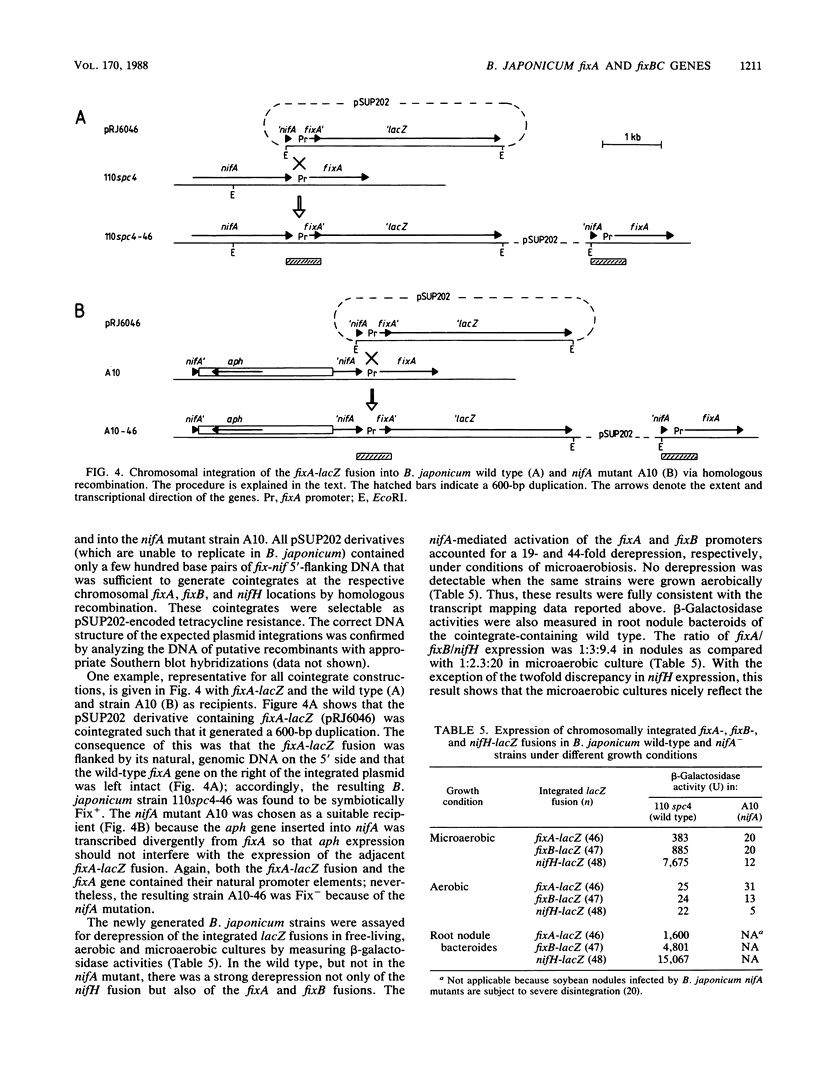
The transcriptional start site of the Bradyrhizobium japonicum fixBC operon was identified by nuclease S1 mapping. It was located approximately 700 base pairs upstream of fixB and was preceded by a promoter sequence that showed strong homology to the B. japonicum fixA promoter and thus to the general nif consensus promoter sequence. Further transcript mapping experiments revealed that fixA and fixBC transcription in B. japonicum strictly depended on the presence of the regulatory gene nifA and on low oxygen partial pressure. Consistent with these data, chromosomally integrated fixA- and fixB-lacZ fusions expressed beta-galactosidase activity only in the wild type but not in a nifA mutant and only under microaerobic but not aerobic growth conditions. The presence of nifA accounted for a 19-fold and 44-fold activation of the fixA and fixB promoters, respectively. These results show that the fixA and fixBC genes are regulated in a way similar to that of the nitrogenase genes nifH and nifDK. A very peculiar finding was that the fixA and fixB promoters, when they were located on plasmids, could hardly be activated by the NifA protein, irrespective of whether this was tested in Escherichia coli or B. japonicum backgrounds. This is in clear contrast to the situation with nifH and nifD promoters.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Morales A., Betancourt-Alvarez M., Kaluza K., Hennecke H. Activation of the Bradyrhizobium japonicum nifH and nifDK operons is dependent on promoter-upstream DNA sequences. Nucleic Acids Res. 1986 May 27;14(10):4207–4227. doi: 10.1093/nar/14.10.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Morales A., Hennecke H. Expression of Rhizobium japonicum nifH and nifDK operons can be activated by the Klebsiella pneumonia nifA protein but not by the product of ntrC. Mol Gen Genet. 1985;199(2):306–314. doi: 10.1007/BF00330273. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Better M., Ditta G., Helinski D. R. Deletion analysis of Rhizobium meliloti symbiotic promoters. EMBO J. 1985 Oct;4(10):2419–2424. doi: 10.1002/j.1460-2075.1985.tb03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Lewis B., Corbin D., Ditta G., Helinski D. R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell. 1983 Dec;35(2 Pt 1):479–485. doi: 10.1016/0092-8674(83)90181-2. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Buck M., Cannon W. Frameshifts close to the Klebsiella pneumoniae nifH promoter prevent multicopy inhibition by hybrid nifH plasmids. Mol Gen Genet. 1987 May;207(2-3):492–498. doi: 10.1007/BF00331620. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Corbin D., Barran L., Ditta G. Organization and expression of Rhizobium meliloti nitrogen fixation genes. Proc Natl Acad Sci U S A. 1983 May;80(10):3005–3009. doi: 10.1073/pnas.80.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J. Determination of fragment order through partial digests and multiple enzyme digests. Methods Enzymol. 1980;65(1):449–467. doi: 10.1016/s0076-6879(80)65055-1. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R., Kennedy C., Kondorosi A., Krishnapillai V., Merrick M. Complementation analysis of Klebsiella pneumoniae mutants defective in nitrogen fixation. Mol Gen Genet. 1977 Nov 29;157(2):189–198. doi: 10.1007/BF00267397. [DOI] [PubMed] [Google Scholar]
- Donald R. G., Nees D. W., Raymond C. K., Loroch A. I., Ludwig R. A. Characterization of three genomic loci encoding Rhizobium sp. strain ORS571 N2 fixation genes. J Bacteriol. 1986 Jan;165(1):72–81. doi: 10.1128/jb.165.1.72-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusha I., Kovalenko S., Banfalvi Z., Kondorosi A. Rhizobium meliloti insertion element ISRm2 and its use for identification of the fixX gene. J Bacteriol. 1987 Apr;169(4):1403–1409. doi: 10.1128/jb.169.4.1403-1409.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl C. D., Ronson C. W., Ausubel F. M. Genetic and structural analysis of the Rhizobium meliloti fixA, fixB, fixC, and fixX genes. J Bacteriol. 1987 Mar;169(3):1127–1136. doi: 10.1128/jb.169.3.1127-1136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H M, Hennecke H. Direct response of Bradyrhizobium japonicum nifA-mediated nif gene regulation to cellular oxygen status. Mol Gen Genet. 1987 Oct;209(3):621–626. doi: 10.1007/BF00331174. [DOI] [PubMed] [Google Scholar]
- Fischer H. M., Alvarez-Morales A., Hennecke H. The pleiotropic nature of symbiotic regulatory mutants: Bradyrhizobium japonicum nifA gene is involved in control of nif gene expression and formation of determinate symbiosis. EMBO J. 1986 Jun;5(6):1165–1173. doi: 10.1002/j.1460-2075.1986.tb04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönger P., Manian S. S., Reiländer H., O'Connell M., Priefer U. B., Pühler A. Organization and partial sequence of a DNA region of the Rhizobium leguminosarum symbiotic plasmid pRL6JI containing the genes fixABC, nifA, nifB and a novel open reading frame. Nucleic Acids Res. 1987 Jan 12;15(1):31–49. doi: 10.1093/nar/15.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussin G. N., Ronson C. W., Ausubel F. M. Regulation of nitrogen fixation genes. Annu Rev Genet. 1986;20:567–591. doi: 10.1146/annurev.ge.20.120186.003031. [DOI] [PubMed] [Google Scholar]
- Iismaa S. E., Watson J. M. A gene upstream of the Rhizobium trifolii nifA gene encodes a ferredoxin-like protein. Nucleic Acids Res. 1987 Apr 10;15(7):3180–3180. doi: 10.1093/nar/15.7.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P. D., Maier R. J. Inhibition of hydrogenase synthesis by DNA gyrase inhibitors in Bradyrhizobium japonicum. J Bacteriol. 1987 Jun;169(6):2708–2712. doi: 10.1128/jb.169.6.2708-2712.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensburger B., Hennecke H. RNA polymerase from Rhizobium japonicum. Arch Microbiol. 1983 Aug;135(2):103–109. doi: 10.1007/BF00408017. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell. 1986 Jun 20;45(6):785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Reznikoff W. S. The functional differences in the inverted repeats of Tn5 are caused by a single base pair nonhomology. Cell. 1981 Jan;23(1):191–199. doi: 10.1016/0092-8674(81)90284-1. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto W. W., Zimmerman J. L., Sundaresan V., Ausubel F. M. A Rhizobium meliloti symbiotic regulatory gene. Cell. 1984 Apr;36(4):1035–1043. doi: 10.1016/0092-8674(84)90053-9. [DOI] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]