Abstract
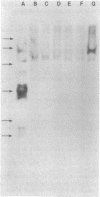
A gene library from the DNA of Coxiella burnetii has been constructed in the cosmid vector pHC79. A particular clone, pJB196, reacted strongly with Coxiella-specific antibodies elicited in a number of different species of animals. This clone produced two abundant C. burnetii-specific polypeptides, a 14-kilodalton nonimmunoreactive protein and a 62-kilodalton immunoreactive protein. Sequencing identified two open reading frames, encoding polypeptides of 10.5 and 58.3 kilodaltons. The only transcriptional control element observed on the 5' side of the initiation codon resembled a heat shock promoter. This heat shock promoter was functionally regulated in Escherichia coli, since both proteins were produced by growth conditions at 37 degrees C and neither protein was detected at 23 degrees C. There were four sequences from the literature that were highly homologous (greater than 50%) to the 62-kilodalton protein from C. burnetii. Three were from Mycobacterium species and represent the immunodominant antigen of this genus. The other was from E. coli, detected as a gene that complements or suppresses a temperature-sensitive RNase activity. Since the recombinant protein was immunogenic, it may serve as an efficacious vaccine against C. burnetii and other pathogenic microorganisms that express the conserved antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A., Rose J. K. Structural requirements of a membrane-spanning domain for protein anchoring and cell surface transport. Cell. 1985 Jul;41(3):1007–1015. doi: 10.1016/s0092-8674(85)80081-7. [DOI] [PubMed] [Google Scholar]
- Amano K., Williams J. C., McCaul T. F., Peacock M. G. Biochemical and immunological properties of Coxiella burnetii cell wall and peptidoglycan-protein complex fractions. J Bacteriol. 1984 Dec;160(3):982–988. doi: 10.1128/jb.160.3.982-988.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher M. S., Berman M. A., Ruppanner R. Initial clinical and immunologic evaluation of a new phase I Q fever vaccine and skin test in humans. J Infect Dis. 1983 Aug;148(2):214–222. doi: 10.1093/infdis/148.2.214. [DOI] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Immunological screening method to detect specific translation products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2746–2749. doi: 10.1073/pnas.75.6.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Nomaguchi H., Anderson D. C., Young R. A., Gillis T. P., Britton W. J., Ivanyi J., Kolk A. H., Closs O., Bloom B. R. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect Immun. 1987 Apr;55(4):1000–1003. doi: 10.1128/iai.55.4.1000-1003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P. K., Ono M., Kuwano M., Kung H. Cloning, sequence analysis, and expression of alteration of the mRNA stability gene (ams+) of Escherichia coli. J Bacteriol. 1985 Jan;161(1):446–449. doi: 10.1128/jb.161.1.446-449.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damrow T. A., Williams J. C., Waag D. M. Suppression of in vitro lymphocyte proliferation in C57BL/10 ScN mice vaccinated with phase I Coxiella burnetii. Infect Immun. 1985 Jan;47(1):149–156. doi: 10.1128/iai.47.1.149-156.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Miller R. A., Young D. B., Khanolkar S. R., Buchanan T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun. 1985 Aug;49(2):371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough R. C., 3rd, Ormsbee R. A., Peacock M., Rogers W. R., Bennetts R. W., Raaf J., Krause A., Gardner C. Q fever endocarditis in the United States. Ann Intern Med. 1979 Sep;91(3):400–402. doi: 10.7326/0003-4819-91-3-400. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Oaks E. V., Stover C. K., Rice R. M. Molecular cloning and expression of Rickettsia tsutsugamushi genes for two major protein antigens in Escherichia coli. Infect Immun. 1987 May;55(5):1156–1162. doi: 10.1128/iai.55.5.1156-1162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock M. G., Philip R. N., Williams J. C., Faulkner R. S. Serological evaluation of O fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infect Immun. 1983 Sep;41(3):1089–1098. doi: 10.1128/iai.41.3.1089-1098.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Codon usage in regulatory genes in Escherichia coli does not reflect selection for 'rare' codons. Nucleic Acids Res. 1986 Oct 10;14(19):7737–7749. doi: 10.1093/nar/14.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Pedersen V. B., Samra Z. An antigen common to a wide range of bacteria. 2. A biochemical study of a "common antigen" from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Oct;88(5):253–260. doi: 10.1111/j.1699-0463.1980.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Samra Z. An antigen common to a wide range of bacteria. I. The isolation of a 'common antigen' from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Jun;88(3):143–149. doi: 10.1111/j.1699-0463.1980.tb02620.x. [DOI] [PubMed] [Google Scholar]
- Spicer D. S., DeSanctis A. N. Preparation of phase 1Q fever antigen suitable for vaccine use. Appl Environ Microbiol. 1976 Jul;32(1):85–88. doi: 10.1128/aem.32.1.85-88.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. Overlapping deletion in two spontaneous phase variants of Coxiella burnetii. J Gen Microbiol. 1986 Sep;132(9):2587–2594. doi: 10.1099/00221287-132-9-2587. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Cantrell J. L. Biological and immunological properties of Coxiella burnetii vaccines in C57BL/10ScN endotoxin-nonresponder mice. Infect Immun. 1982 Mar;35(3):1091–1102. doi: 10.1128/iai.35.3.1091-1102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Damrow T. A., Waag D. M., Amano K. Characterization of a phase I Coxiella burnetii chloroform-methanol residue vaccine that induces active immunity against Q fever in C57BL/10 ScN mice. Infect Immun. 1986 Mar;51(3):851–858. doi: 10.1128/iai.51.3.851-858.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peacock M. G., McCaul T. F. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun. 1981 May;32(2):840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Thomas L. A., Peacock M. G. Humoral immune response to Q fever: enzyme-linked immunosorbent assay antibody response to Coxiella burnetii in experimentally infected guinea pigs. J Clin Microbiol. 1986 Dec;24(6):935–939. doi: 10.1128/jcm.24.6.935-939.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Thomas L. A., Peacock M. G. Identification of phase-specific antigenic fractions of Coxiella burnetti by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Dec;24(6):929–934. doi: 10.1128/jcm.24.6.929-934.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]