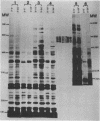
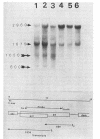
Abstract
We have analyzed early and late T4 gene expression at the levels of transcription and translation in rpoH+ (sigma 32+) and rpoH mutant cells infected under heat shock conditions. We found, as expected, that Escherichia coli cells must be adapted before infection to high temperature by the heat shock response to allow early T4 transcription, subsequent late gene expression, and progeny production at 42 degrees C. Unexpectedly, we found in addition that when rpoH mutant (sigma 32 mutant) cells were shifted from 30 to 42 degrees C 10 min after infection, late T4 genes were not expressed, even though DNA synthesis appeared to be normal.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfort M. Anomalous behavior of bacteriophage lambda polypeptides in polyacrylamide gels: resolution, identification, and control of the lambda rex gene product. J Virol. 1978 Oct;28(1):270–278. doi: 10.1128/jvi.28.1.270-278.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheley S., Anderson R. A reproducible microanalytical method for the detection of specific RNA sequences by dot-blot hybridization. Anal Biochem. 1984 Feb;137(1):15–19. doi: 10.1016/0003-2697(84)90339-7. [DOI] [PubMed] [Google Scholar]
- Gorski K., Roch J. M., Prentki P., Krisch H. M. The stability of bacteriophage T4 gene 32 mRNA: a 5' leader sequence that can stabilize mRNA transcripts. Cell. 1985 Dec;43(2 Pt 1):461–469. doi: 10.1016/0092-8674(85)90176-x. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Zhou Y. N., Gross C., Heilig J., Christie G. E., Calendar R. Mutations in the rpoH (htpR) gene of Escherichia coli K-12 phenotypically suppress a temperature-sensitive mutant defective in the sigma 70 subunit of RNA polymerase. J Bacteriol. 1985 Mar;161(3):939–943. doi: 10.1128/jb.161.3.939-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruidl M. E., Mosig G. Sequence and transcripts of the bacteriophage T4 DNA repair gene uvsY. Genetics. 1986 Dec;114(4):1061–1079. doi: 10.1093/genetics/114.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homyk T., Jr, Weil J. Deletion analysis of two nonessential regions of the T4 genome. Virology. 1974 Oct;61(2):505–523. doi: 10.1016/0042-6822(74)90286-4. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Geiduschek E. P. Defining a bacteriophage T4 late promoter: bacteriophage T4 gene 55 protein suffices for directing late promoter recognition. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5101–5105. doi: 10.1073/pnas.81.16.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Mosig G. Regulation of a new bacteriophage T4 gene, 69, that spans an origin of DNA replication. EMBO J. 1984 Dec 1;3(12):2863–2871. doi: 10.1002/j.1460-2075.1984.tb02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G., Bowden D. W., Bock S. E. coli DNA polymerase I and other host functions participate in T4 DNA replication and recombination. Nat New Biol. 1972 Nov 1;240(96):12–16. doi: 10.1038/newbio240012a0. [DOI] [PubMed] [Google Scholar]
- Mosig G., Breschkin A. M. Genetic evidence for an additional function of phage T4 gene 32 protein: interaction with ligase. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1226–1230. doi: 10.1073/pnas.72.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa T., Yura T. Effects of reduced amount of RNA polymerase sigma factor on gene expression and growth of Escherichia coli: studies of the rpoD450 (amber) mutation. Mol Gen Genet. 1981;184(2):166–173. doi: 10.1007/BF00272900. [DOI] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Coupling of late transcription to viral replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):85–102. doi: 10.1016/0022-2836(70)90447-x. [DOI] [PubMed] [Google Scholar]
- Skelly S., Coleman T., Fu C. F., Brot N., Weissbach H. Correlation between the 32-kDa sigma factor levels and in vitro expression of Escherichia coli heat shock genes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8365–8369. doi: 10.1073/pnas.84.23.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. E., Straus D. B., Grossman A. D., Burton Z. F., Gross C. A., Burgess R. R. Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell. 1984 Sep;38(2):371–381. doi: 10.1016/0092-8674(84)90492-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., Georgopoulos C. Evidence that the two Escherichia coli groE morphogenetic gene products interact in vivo. J Bacteriol. 1982 Mar;149(3):1082–1088. doi: 10.1128/jb.149.3.1082-1088.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S., Mowrey-McKee M. F., Stevens E. J. Induction of the heat shock regulon of Escherichia coli markedly increases production of bacterial viruses at high temperatures. J Virol. 1988 Jan;62(1):234–245. doi: 10.1128/jvi.62.1.234-245.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zograff Y. N. On the role of the Escherichia coli RNA polymerase sigma factor in T4 phage development. Mol Gen Genet. 1981;183(3):557–558. doi: 10.1007/BF00268782. [DOI] [PubMed] [Google Scholar]