Abstract
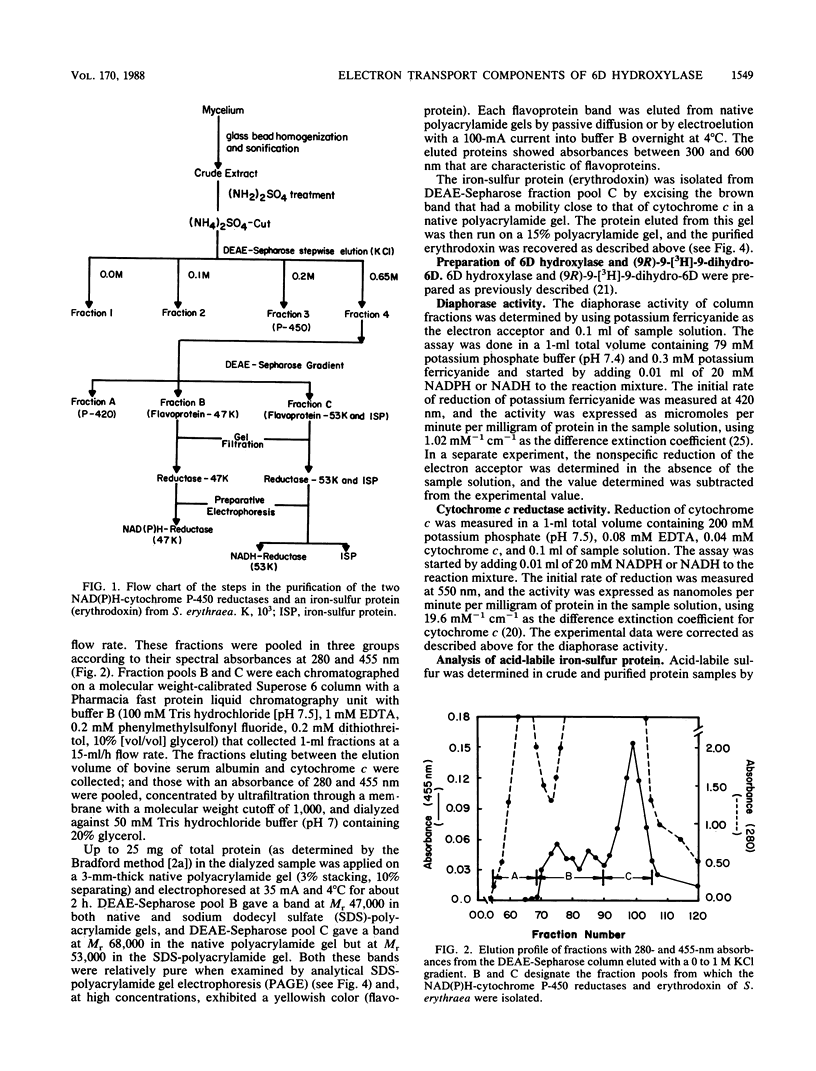
The hydroxylation of 6-deoxyerythronolide B (6D) to erythronolide B, a step in the biosynthesis of the 14-membered macrolide antibiotic erythromycin A by Saccharopolyspora erythraea, is catalyzed by a cytochrome P-450 monooxygenase that requires two electron transport proteins for the function of this terminal hydroxylase (A. Shafiee and C. R. Hutchinson, Biochemistry 26:6204-6210, 1987). Two flavoproteins and an iron-sulfur protein (erythrodoxin) were purified from S. erythraea CA340 and shown to act with 6D hydroxylase to catalyze the hydroxylation of (9R)-[9-3H]9-deoxo-9-hydroxy-6D in vitro in a suitably reconstituted system. The flavoproteins contained flavin adenine dinucleotide and exhibited characteristic absorption maxima at 356 and 456 nm. The one with an Mr of 47,000 showed NADPH-dependent diaphorase and cytochrome c reductase activity, and the other, with an Mr of 53,000 showed NADH-dependent activities of the same two types. Erythrodoxin contained acid-labile sulfur and iron, had an Mr of 27,500, and showed a broad absorption maximum between 394 and 404 nm. The sequence of its first 15 amino acids, except for position 12, was the same as that of the ferredoxin from Mycobacterium smegmatis.
Full text
PDF
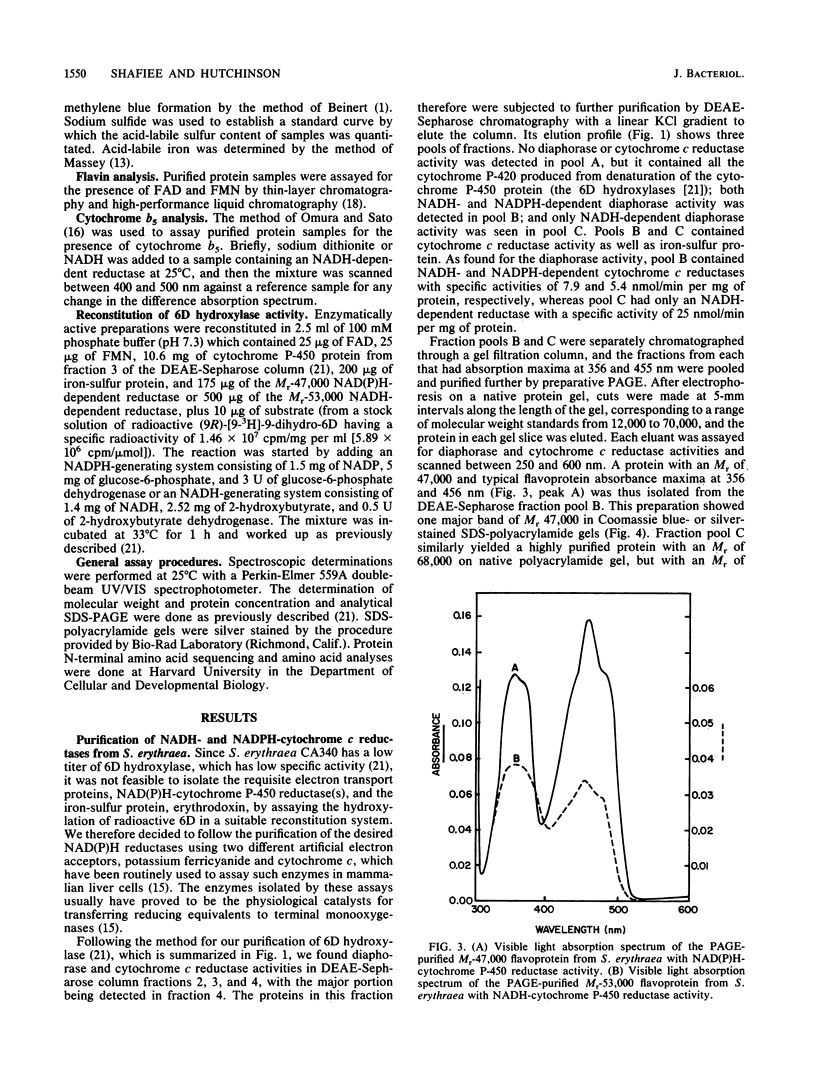


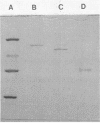
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H. Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron-sulfur proteins. Anal Biochem. 1983 Jun;131(2):373–378. doi: 10.1016/0003-2697(83)90186-0. [DOI] [PubMed] [Google Scholar]
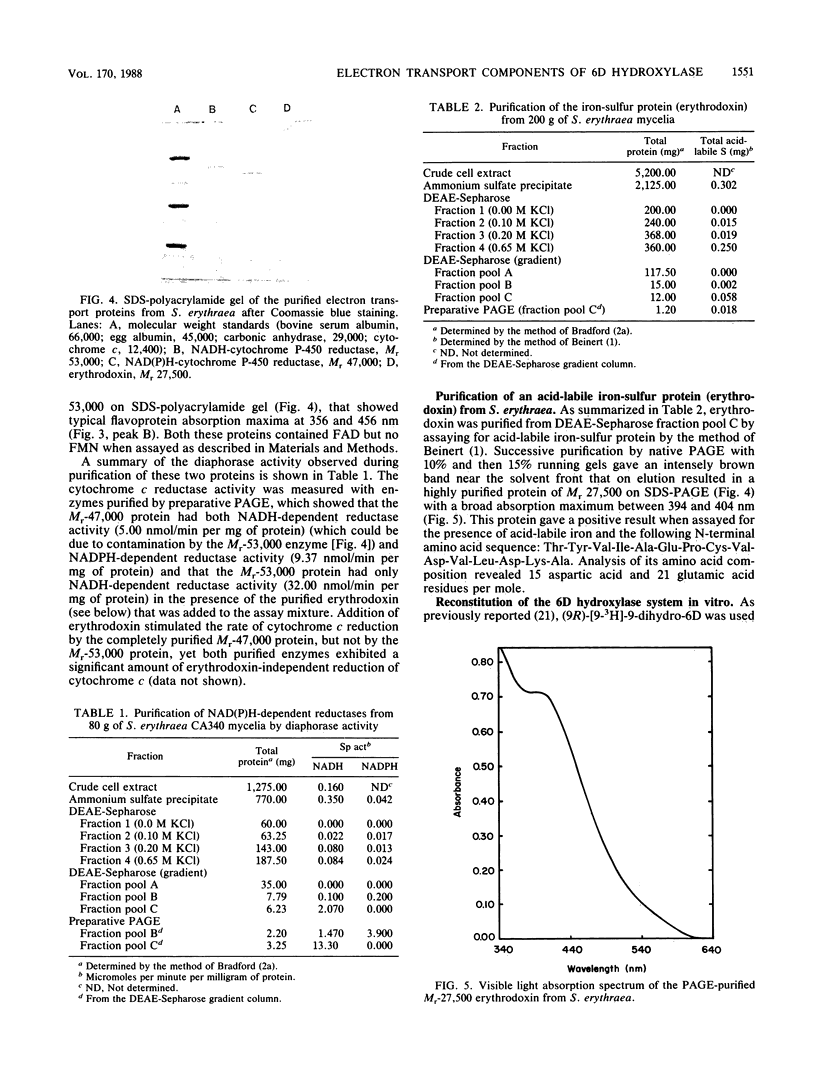
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chu J. W., Kimura T. Studies on adrenal steroid hydroxylases. Molecular and catalytic properties of adrenodoxin reductase (a flavoprotein). J Biol Chem. 1973 Mar 25;248(6):2089–2094. [PubMed] [Google Scholar]
- Corcoran J. W., Vygantas A. M. Accumulation of 6-deoxyerythronolide B in a normal strain of Streptomyces erythreus and hydroxylation at carbon 6 of the erythranolide ring system by a soluble noninduced cell-free enzyme system. Biochemistry. 1982 Jan 19;21(2):263–269. doi: 10.1021/bi00531a010. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Dannan G. A., Wright S. T., Martin M. V., Kaminsky L. S. Purification and characterization of liver microsomal cytochromes p-450: electrophoretic, spectral, catalytic, and immunochemical properties and inducibility of eight isozymes isolated from rats treated with phenobarbital or beta-naphthoflavone. Biochemistry. 1982 Nov 9;21(23):6019–6030. doi: 10.1021/bi00266a045. [DOI] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Matsubara H., Imai T., Matsumoto T., Tobari J. Mycobacterium smegmatis ferredoxin: a unique distribution of cysteine residues constructing iron--sulfur clusters. FEBS Lett. 1979 Jul 15;103(2):224–228. doi: 10.1016/0014-5793(79)81332-0. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A., Estabrook R. W. Evidence for the participation of cytochrome b 5 in hepatic microsomal mixed-function oxidation reactions. Arch Biochem Biophys. 1971 Mar;143(1):66–79. doi: 10.1016/0003-9861(71)90186-x. [DOI] [PubMed] [Google Scholar]
- Kawata S., Trzaskos J. M., Gaylor J. L. Microsomal enzymes of cholesterol biosynthesis from lanosterol. Purification and characterization of delta 7-sterol 5-desaturase of rat liver microsomes. J Biol Chem. 1985 Jun 10;260(11):6609–6617. [PubMed] [Google Scholar]
- Koga H., Aramaki H., Yamaguchi E., Takeuchi K., Horiuchi T., Gunsalus I. C. camR, a negative regulator locus of the cytochrome P-450cam hydroxylase operon. J Bacteriol. 1986 Jun;166(3):1089–1095. doi: 10.1128/jb.166.3.1089-1095.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode E. T., Coon M. J. Enzymatic omega-oxidation. V. Forms of Pseudomonas oleovorans rubredoxin containing one or two iron atoms: structure and function in omega-hydroxylation. J Biol Chem. 1971 Feb 10;246(3):791–802. [PubMed] [Google Scholar]
- MASSEY V. Studies on succinic dehydrogenase. VII. Valency state of the iron in beef heart succinic dehydrogenase. J Biol Chem. 1957 Dec;229(2):763–770. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Okamura T., John M. E., Zuber M. X., Simpson E. R., Waterman M. R. Molecular cloning and amino acid sequence of the precursor form of bovine adrenodoxin: evidence for a previously unidentified COOH-terminal peptide. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5705–5709. doi: 10.1073/pnas.82.17.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter T. D., Wilson T. E., Kasper C. B. Expression of a functional 78,000 dalton mammalian flavoprotein, NADPH-cytochrome P-450 oxidoreductase, in Escherichia coli. Arch Biochem Biophys. 1987 Apr;254(1):353–367. doi: 10.1016/0003-9861(87)90111-1. [DOI] [PubMed] [Google Scholar]
- Romesser J. A., O'Keefe D. P. Induction of cytochrome P-450-dependent sulfonylurea metabolism in Streptomyces griseolus. Biochem Biophys Res Commun. 1986 Oct 30;140(2):650–659. doi: 10.1016/0006-291x(86)90781-3. [DOI] [PubMed] [Google Scholar]
- SCHELLENBERG K. A., HELLERMAN L. Oxidation of reduced diphosphopyridine nucleotide. J Biol Chem. 1958 Mar;231(1):547–556. [PubMed] [Google Scholar]
- Shafiee A., Hutchinson C. R. Macrolide antibiotic biosynthesis: isolation and properties of two forms of 6-deoxyerythronolide B hydroxylase from Saccharopolyspora erythraea (Streptomyces erythreus). Biochemistry. 1987 Sep 22;26(19):6204–6210. doi: 10.1021/bi00393a037. [DOI] [PubMed] [Google Scholar]
- Thiede M. A., Ozols J., Strittmatter P. Construction and sequence of cDNA for rat liver stearyl coenzyme A desaturase. J Biol Chem. 1986 Oct 5;261(28):13230–13235. [PubMed] [Google Scholar]
- Trzaskos J. M., Bowen W. D., Shafiee A., Fischer R. T., Gaylor J. L. Cytochrome P-450-dependent oxidation of lanosterol in cholesterol biosynthesis. Microsomal electron transport and C-32 demethylation. J Biol Chem. 1984 Nov 10;259(21):13402–13412. [PubMed] [Google Scholar]
- Tsai R. L., Gunsalus I. C., Dus K. Composition and structure of camphor hydroxylase components and homology between putidaredoxin and adrenodoxin. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1300–1306. doi: 10.1016/0006-291x(71)90160-4. [DOI] [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J Biol Chem. 1965 Nov;240(11):4509–4514. [PubMed] [Google Scholar]