Abstract
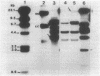
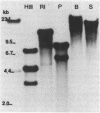
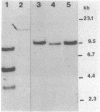
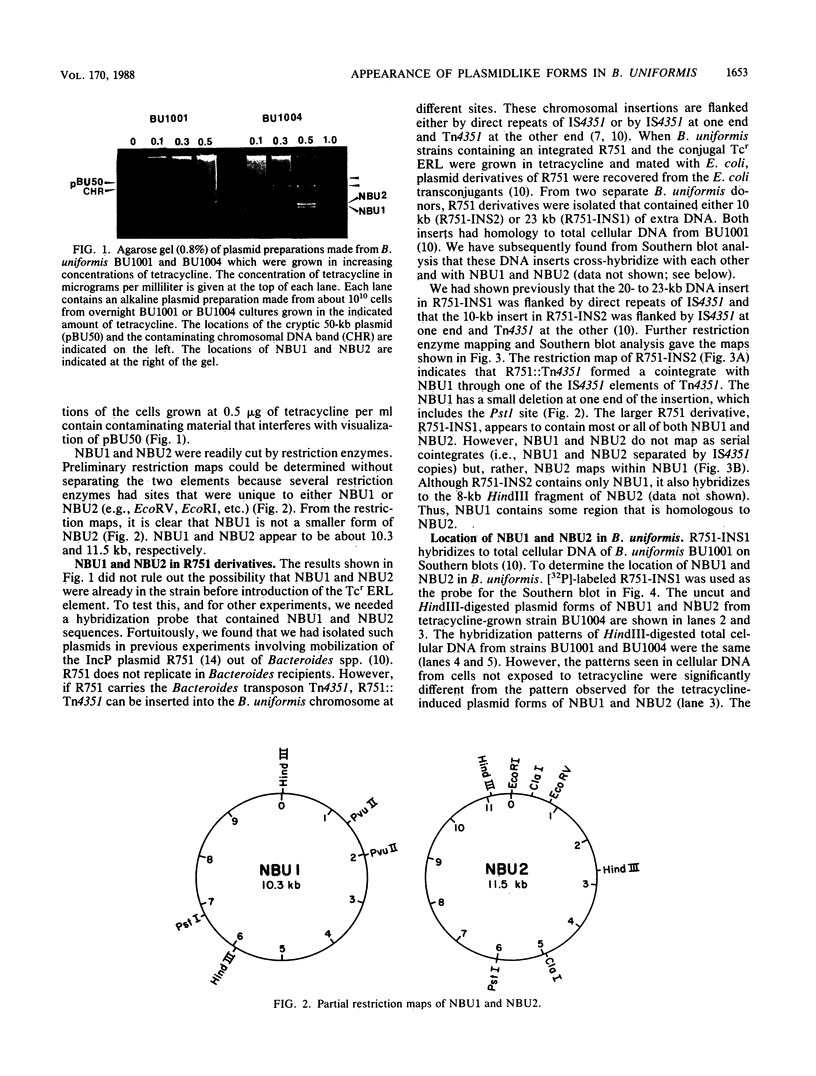
Some human colonic Bacteroides strains carry conjugal tetracycline resistance (Tcr) elements, which are thought to be chromosomal. We have found that some of these Tcr elements can mediate the appearance of plasmidlike forms in Bacteroides uniformis 0061. When B. uniformis 0061, containing a conjugal Tcr element designated Tcr ERL, was grown in medium containing tetracycline (1 microgram/ml), two circular DNA forms were found in the alkaline plasmid preparations: NBU1 (10.3 +/- 0.5 kilobases) and NBU2 (11.5 +/- 0.5 kilobases). Restriction analysis of NBU1 and NBU2 showed that they were not identical, although Southern blot analysis indicated that they did contain some region(s) of homology. Results of Southern blot analysis also demonstrated that both NBU1 and NBU2 were normally integrated in the chromosome of B. uniformis or in some undetected large plasmid. Although we were unable to determine the exact structure and location of the integrated forms of NBU1 and NBU2 in B. uniformis, they appear to be in close proximity to each other. Neither NBU1 or NBU2 could be detected as a plasmidlike form in cells exposed to UV light, thymidine starvation, mitomycin C, or autoclaved chlortetracycline (50 micrograms/ml). Four conjugal Tcr elements other than the Tcr ERL element were able to mediate the appearance of NBU1 alone, and two Tcr elements did not mediate the excision of either NBU1 or NBU2. Three strains from different Bacteroides species contained some DNA sequences which had homology to NBU1 and NBU2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy M. H., Tally F. P. Mechanisms of drug-resistance transfer in Bacteroides fragilis. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):59–75. doi: 10.1093/jac/8.suppl_d.59. [DOI] [PubMed] [Google Scholar]
- Mays T. D., Smith C. J., Welch R. A., Delfini C., Macrina F. L. Novel antibiotic resistance transfer in Bacteroides. Antimicrob Agents Chemother. 1982 Jan;21(1):110–118. doi: 10.1128/aac.21.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G., Sebald M., Fayolle F. Common regulatory mechanism of expression and conjugative ability of a tetracycline resistance plasmid in Bacteroides fragilis. Nature. 1979 Apr 12;278(5705):657–659. doi: 10.1038/278657a0. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Gardner J. F., Salyers A. A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986 Mar;165(3):929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Guthrie E. P., Salyers A. A. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986 Jun;166(3):959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Guthrie E. P., Salyers A. A., Gardner J. F. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol. 1985 May;162(2):626–632. doi: 10.1128/jb.162.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Salyers A. A. Facilitated transfer of IncP beta R751 derivatives from the chromosome of Bacteroides uniformis to Escherichia coli recipients by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1987 Jul;169(7):3160–3167. doi: 10.1128/jb.169.7.3160-3167.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Macrina F. L. Large transmissible clindamycin resistance plasmid in Bacteroides ovatus. J Bacteriol. 1984 May;158(2):739–741. doi: 10.1128/jb.158.2.739-741.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Spiegel H. Transposition of Tn4551 in Bacteroides fragilis: identification and properties of a new transposon from Bacteroides spp. J Bacteriol. 1987 Aug;169(8):3450–3457. doi: 10.1128/jb.169.8.3450-3457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Welch R. A., Macrina F. L. Two independent conjugal transfer systems operating in Bacteroides fragilis V479-1. J Bacteriol. 1982 Jul;151(1):281–287. doi: 10.1128/jb.151.1.281-287.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. M., Smith C. A. Incompatibility group P plasmids: genetics, evolution, and use in genetic manipulation. Annu Rev Microbiol. 1987;41:77–101. doi: 10.1146/annurev.mi.41.100187.000453. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Macrina F. L. Physical characterization of Bacteroides fragilis R plasmid pBF4. J Bacteriol. 1981 Feb;145(2):867–872. doi: 10.1128/jb.145.2.867-872.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]