Abstract
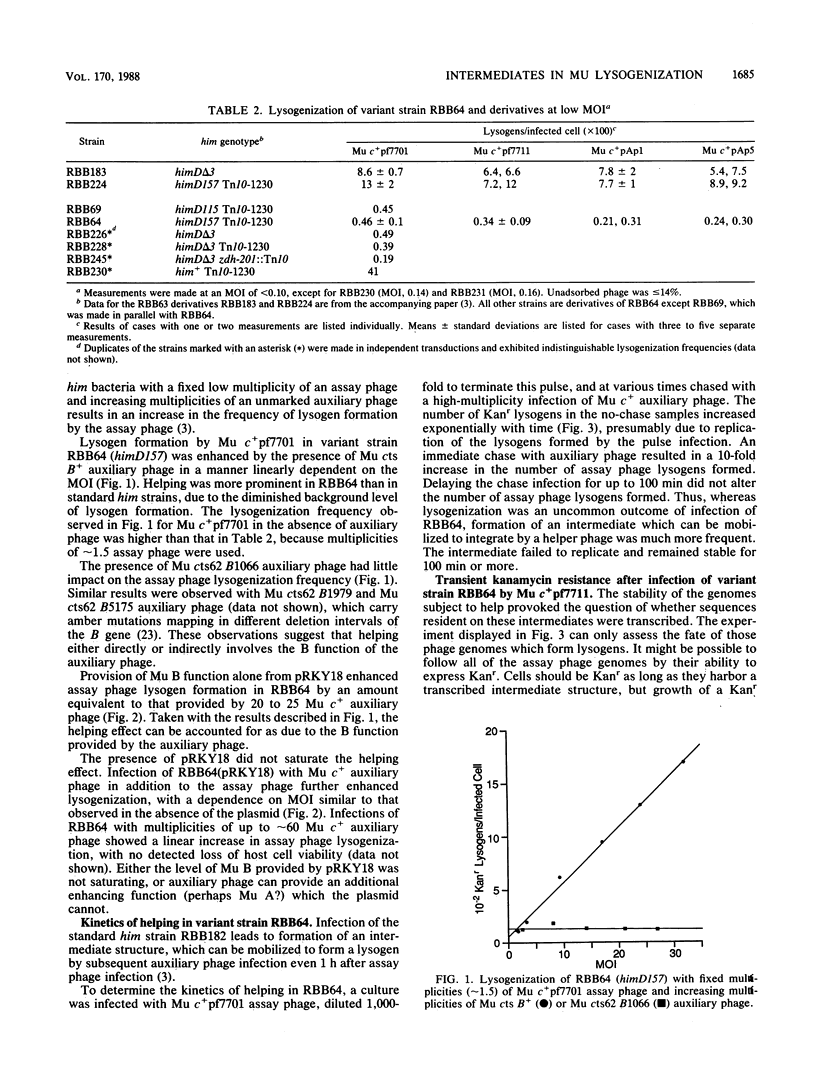
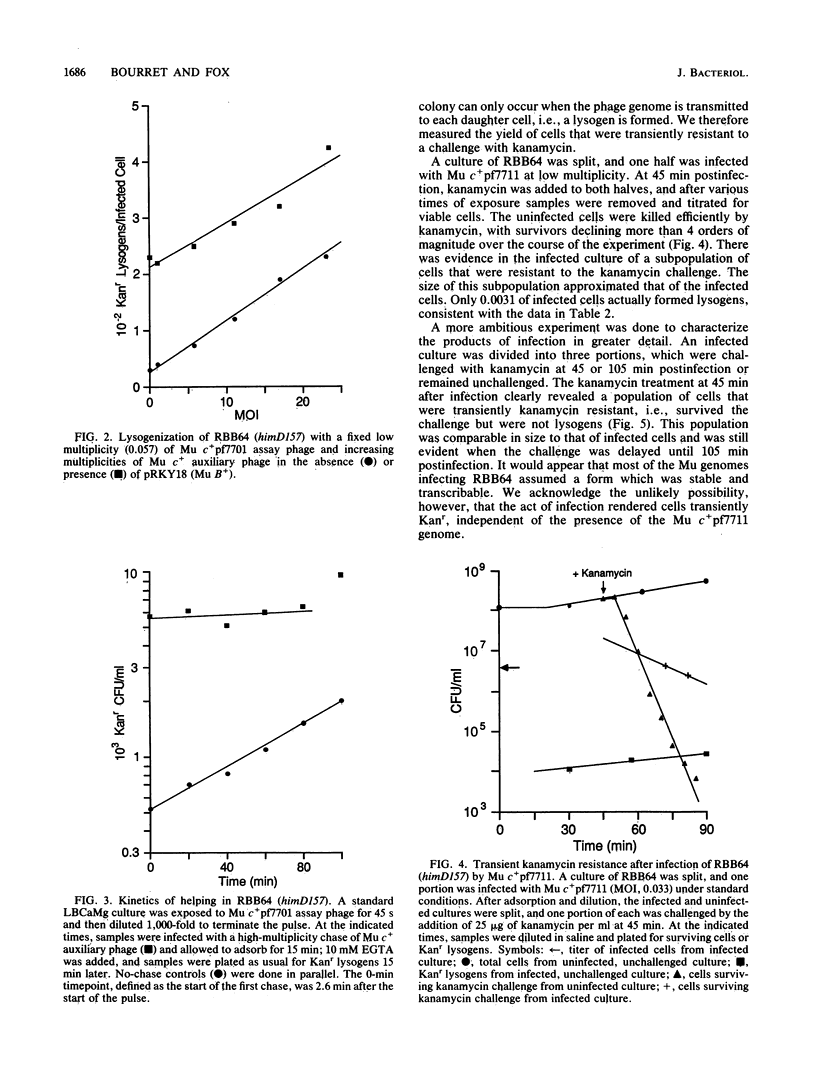
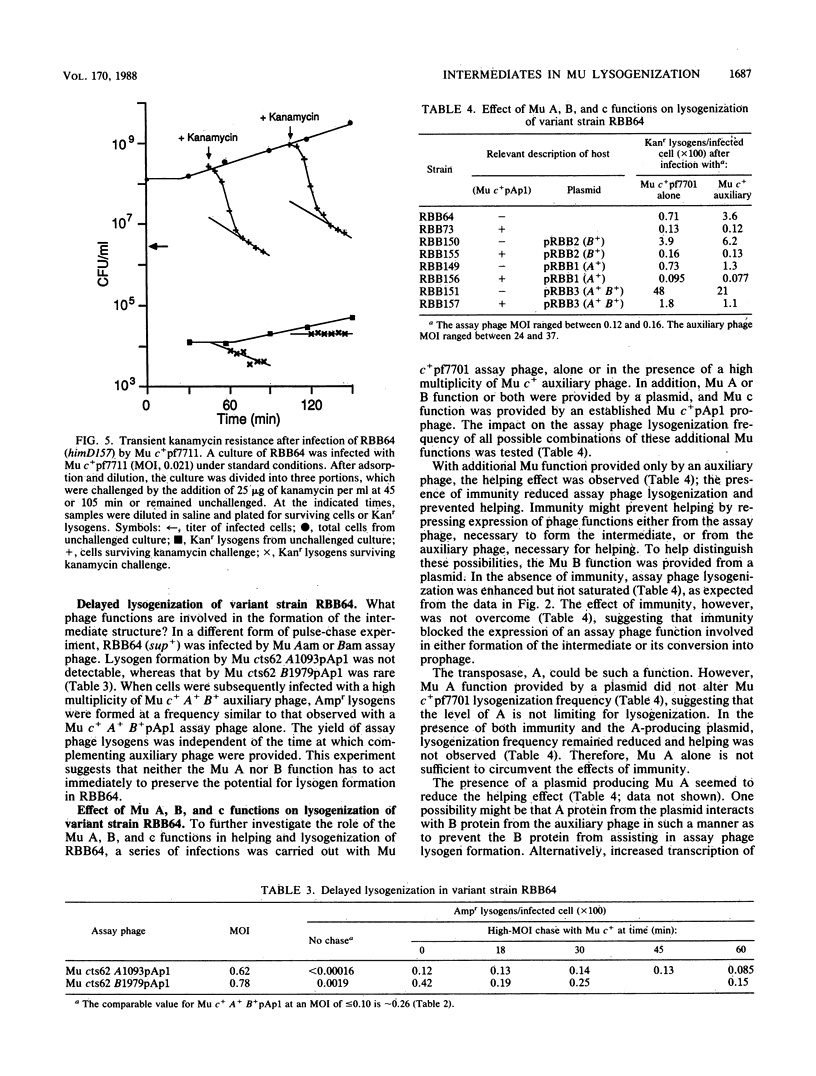
Characterization of a putative intermediate in the Mu lysogenization pathway is possible in a variant Escherichia coli himD strain which exhibits greatly diminished lysogen formation. In this strain, most infecting Mu genomes form stable, transcribable, nonreplicating structures. Many of these genomes can be mobilized to form lysogens by a second Mu infection, which can be delayed by at least 100 min. This intermediate structure can be formed in the absence of Mu A or B function. We suggest that the inferred intermediate could be the previously reported protein-linked circular form of the Mu genome. Providing Mu B function from a plasmid enhances Mu lysogenization in this him strain, and the enhancement is much greater when both Mu A and B functions are provided.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Bourret R. B., Fox M. S. Lysogenization of Escherichia coli him+, himA, and himD hosts by bacteriophage Mu. J Bacteriol. 1988 Apr;170(4):1672–1682. doi: 10.1128/jb.170.4.1672-1682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G., Gloor G., Miller J. L., Kennedy D. L., Giddens E. B., Nagainis C. R. Transposition of bacteriophage mu DNA: expression of the A and B proteins from lambda pL and analysis of infecting mu DNA. Cold Spring Harb Symp Quant Biol. 1984;49:279–284. doi: 10.1101/sqb.1984.049.01.033. [DOI] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K. Mechanism of transposition of bacteriophage Mu: structure of a transposition intermediate. Cell. 1985 Jul;41(3):867–876. doi: 10.1016/s0092-8674(85)80067-2. [DOI] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K. Transposition of Mu DNA: joining of Mu to target DNA can be uncoupled from cleavage at the ends of Mu. Cell. 1987 Nov 6;51(3):493–501. doi: 10.1016/0092-8674(87)90645-3. [DOI] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi M., Mizuuchi K. Site-specific recognition of the bacteriophage Mu ends by the Mu A protein. Cell. 1984 Dec;39(2 Pt 1):387–394. doi: 10.1016/0092-8674(84)90017-5. [DOI] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel-Tsipis J., Fox M. S., Botstein D. Generalized transduction by bacteriophage P22 in Salmonella typhimurium. II. Mechanism of integration of transducing DNA. J Mol Biol. 1972 Nov 14;71(2):449–469. doi: 10.1016/0022-2836(72)90362-2. [DOI] [PubMed] [Google Scholar]
- Flamm E. L., Weisberg R. A. Primary structure of the hip gene of Escherichia coli and of its product, the beta subunit of integration host factor. J Mol Biol. 1985 May 25;183(2):117–128. doi: 10.1016/0022-2836(85)90206-2. [DOI] [PubMed] [Google Scholar]
- Gloor G., Chaconas G. The bacteriophage Mu N gene encodes the 64-kDa virion protein which is injected with, and circularizes, infecting Mu DNA. J Biol Chem. 1986 Dec 15;261(35):16682–16688. [PubMed] [Google Scholar]
- Harshey R. M., Bukhari A. I. Infecting bacteriophage mu DNA forms a circular DNA-protein complex. J Mol Biol. 1983 Jun 25;167(2):427–441. doi: 10.1016/s0022-2836(83)80343-x. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Olivera B. M., Roth J. R. Rec dependence of mu transposition from P22-transduced fragments. J Bacteriol. 1987 Jan;169(1):403–409. doi: 10.1128/jb.169.1.403-409.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Flamm E., Weisberg R. A. An Escherichia coli mutant unable to support site-specific recombination of bacteriophage lambda. J Mol Biol. 1985 May 25;183(2):129–140. doi: 10.1016/0022-2836(85)90207-4. [DOI] [PubMed] [Google Scholar]
- Leach D., Symonds N. The isolation and characterisation of a plaque-forming derivative of bacteriophage Mu carrying a fragment of Tn3 conferring ampicillin resistance. Mol Gen Genet. 1979 May 4;172(2):179–184. doi: 10.1007/BF00268280. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Friedman D. I. An E. coli gene product required for lambda site-specific recombination. Cell. 1980 Jul;20(3):711–719. doi: 10.1016/0092-8674(80)90317-7. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Nash H. A. Direct role of the himA gene product in phage lambda integration. Nature. 1981 Apr 9;290(5806):523–526. doi: 10.1038/290523a0. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Craigie R. Mechanism of bacteriophage mu transposition. Annu Rev Genet. 1986;20:385–429. doi: 10.1146/annurev.ge.20.120186.002125. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K. In vitro transposition of bacteriophage Mu: a biochemical approach to a novel replication reaction. Cell. 1983 Dec;35(3 Pt 2):785–794. doi: 10.1016/0092-8674(83)90111-3. [DOI] [PubMed] [Google Scholar]
- O'Day K., Schultz D., Ericsen W., Rawluk L., Howe M. Correction and refinement of the genetic map of bacteriophage Mu. Virology. 1979 Mar;93(2):320–328. doi: 10.1016/0042-6822(79)90236-8. [DOI] [PubMed] [Google Scholar]
- Ozeki H. Chromosome Fragments Participating in Transduction in Salmonella Typhimurium. Genetics. 1959 May;44(3):457–470. doi: 10.1093/genetics/44.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puspurs A. H., Trun N. J., Reeve J. N. Bacteriophage Mu DNA circularizes following infection of Escherichia coli. EMBO J. 1983;2(3):345–352. doi: 10.1002/j.1460-2075.1983.tb01429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Shore S. H., Howe M. M. Mutants of Escherichia coli defective for replicative transposition of bacteriophage Mu. J Bacteriol. 1986 Sep;167(3):905–919. doi: 10.1128/jb.167.3.905-919.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette M. G., Buch S. J., Chaconas G. Transpososomes: stable protein-DNA complexes involved in the in vitro transposition of bacteriophage Mu DNA. Cell. 1987 Apr 24;49(2):253–262. doi: 10.1016/0092-8674(87)90566-6. [DOI] [PubMed] [Google Scholar]


