Abstract
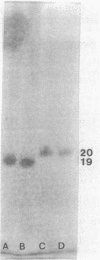
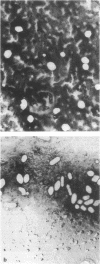
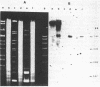
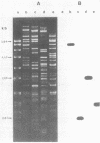
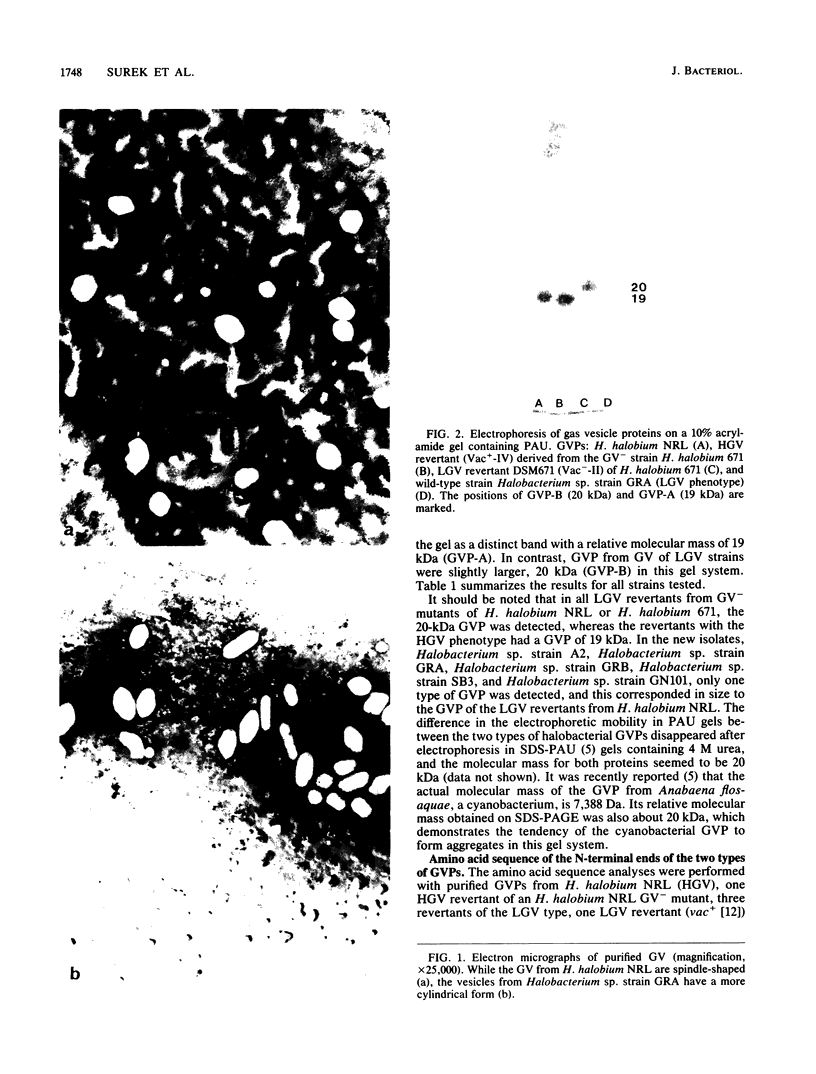
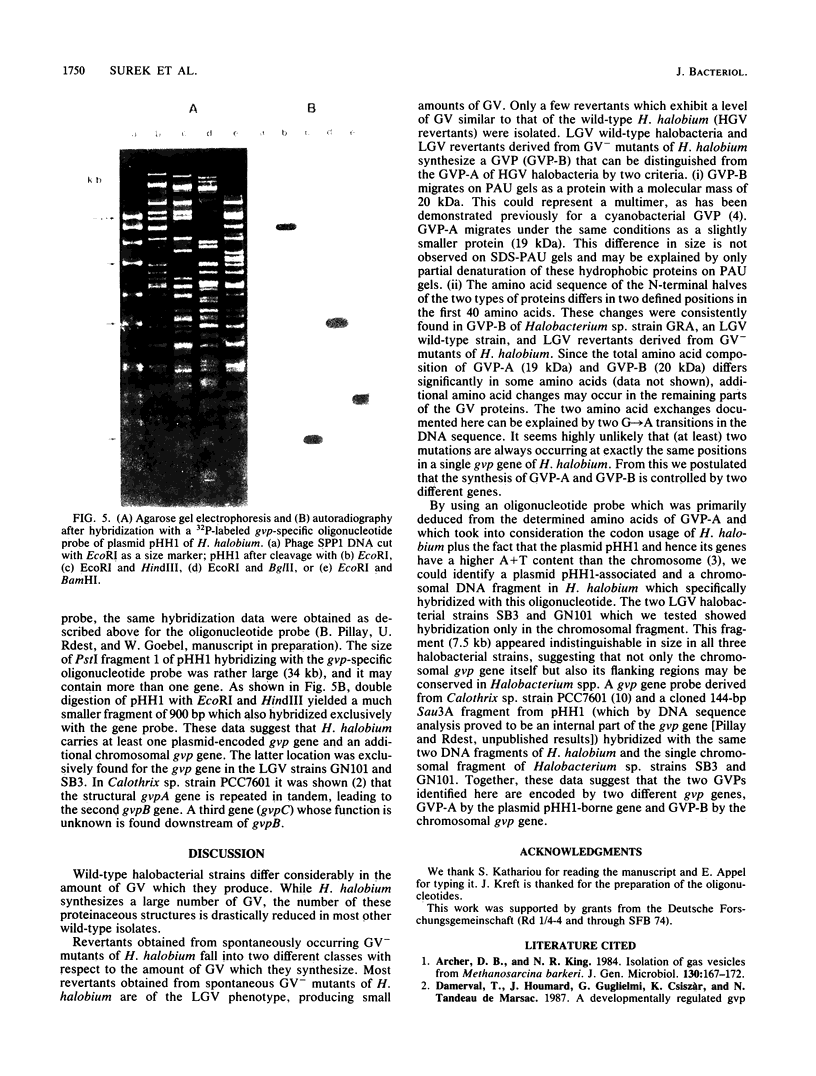
Most halobacteria produce gas vesicles (GV). The well-characterized species Halobacterium halobium and some GV+ revertants of GV- mutants of H. halobium produce large amounts of GV which have a spindlelike shape. Most other GV+ revertants of H. halobium GV- mutants and other recently characterized halobacterial wild-type strains possess GV with a cylindrical form. The number of intact particles in the latter isolates is only 10 to 30% of that of H. halobium. Analysis of GV envelope proteins (GVPs) by electrophoresis on phenol-acetic acid-urea gels showed that the GVP of the highly efficient GV-producing strains migrated faster than the GVP of the low-GV-producing strains. The relative molecular mass of the GVP was estimated to be 19 kilodaltons (kDa) for high-producing strains (GVP-A) and 20 kDa for low-producing strains (GVP-B). Amino acid sequence analysis of the first 40 amino acids of the N-terminal parts of GVP-A and GVP-B indicated that the two proteins differed in two defined positions. GVP-B, in relation to GVP-A, had Gly-7 and Val-28 always replaced by Ser-7 and Ile-28, respectively. These data suggest that at least two different gvp genes exist in H. halobium NRL. This was directly demonstrated by hybridization experiments with gvp-specific DNA probes. A fragment of plasmid pHH1 and a chromosomal fragment of H. halobium hybridized to the probes. Only a chromosomal fragment hybridized to the same gyp probes when both chromosomal and plasmid DNAs from the low-GV-producing halobacterial wild-type strains SB3 and GN101 were examined. These findings support the assumption that GVP-A is expressed by a pHH1-associated gvp gene and GVP-B by a chromosomal gvp gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hayes P. K., Walsby A. E., Walker J. E. Complete amino acid sequence of cyanobacterial gas-vesicle protein indicates a 70-residue molecule that corresponds in size to the crystallographic unit cell. Biochem J. 1986 May 15;236(1):31–36. doi: 10.1042/bj2360031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Characterization of plasmids in halobacteria. J Bacteriol. 1981 Jan;145(1):369–374. doi: 10.1128/jb.145.1.369-374.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Genetic variability in Halobacterium halobium. J Bacteriol. 1981 Jan;145(1):375–381. doi: 10.1128/jb.145.1.375-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Mazel D., Bryant D. A., Houmard J. Molecular cloning and nucleotide sequence of a developmentally regulated gene from the cyanobacterium Calothrix PCC 7601: a gas vesicle protein gene. Nucleic Acids Res. 1985 Oct 25;13(20):7223–7236. doi: 10.1093/nar/13.20.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby A. E. Structure and function of gas vacuoles. Bacteriol Rev. 1972 Mar;36(1):1–32. doi: 10.1128/br.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger G., Klotz G., Goebel W. A large plasmid from Halobacterium halobium carrying genetic information for gas vacuole formation. Plasmid. 1979 Jul;2(3):377–386. doi: 10.1016/0147-619x(79)90021-0. [DOI] [PubMed] [Google Scholar]