Abstract
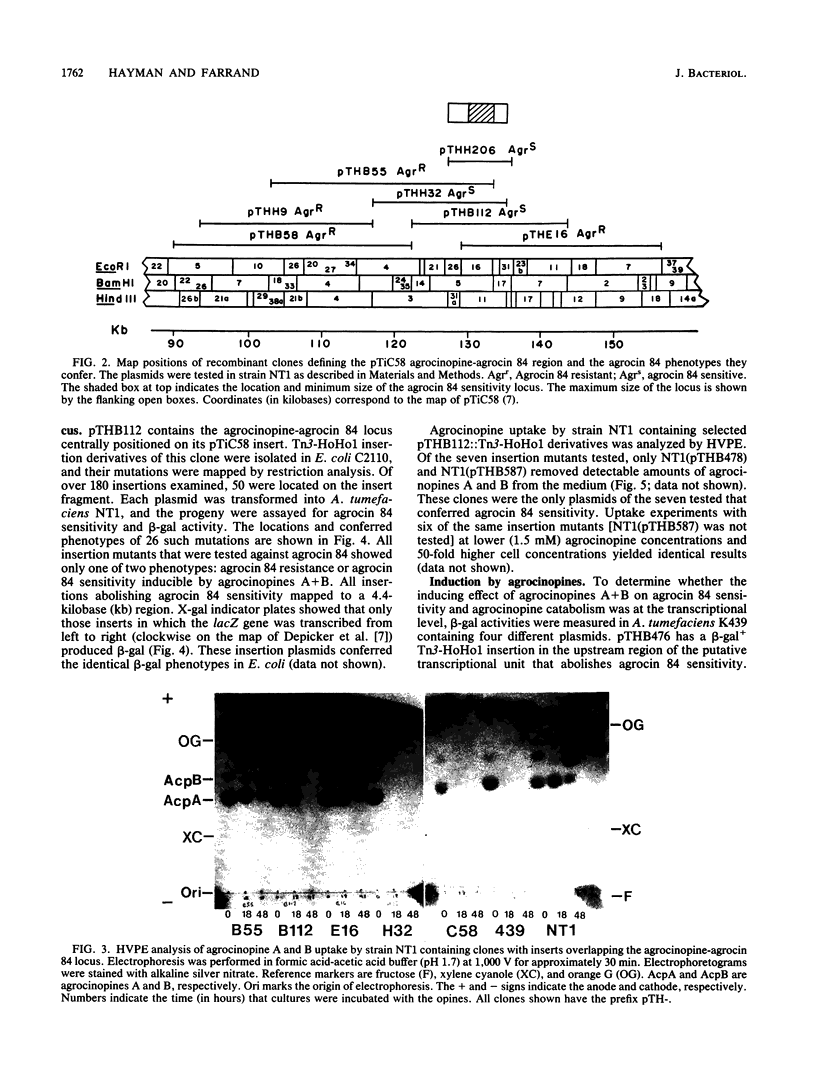
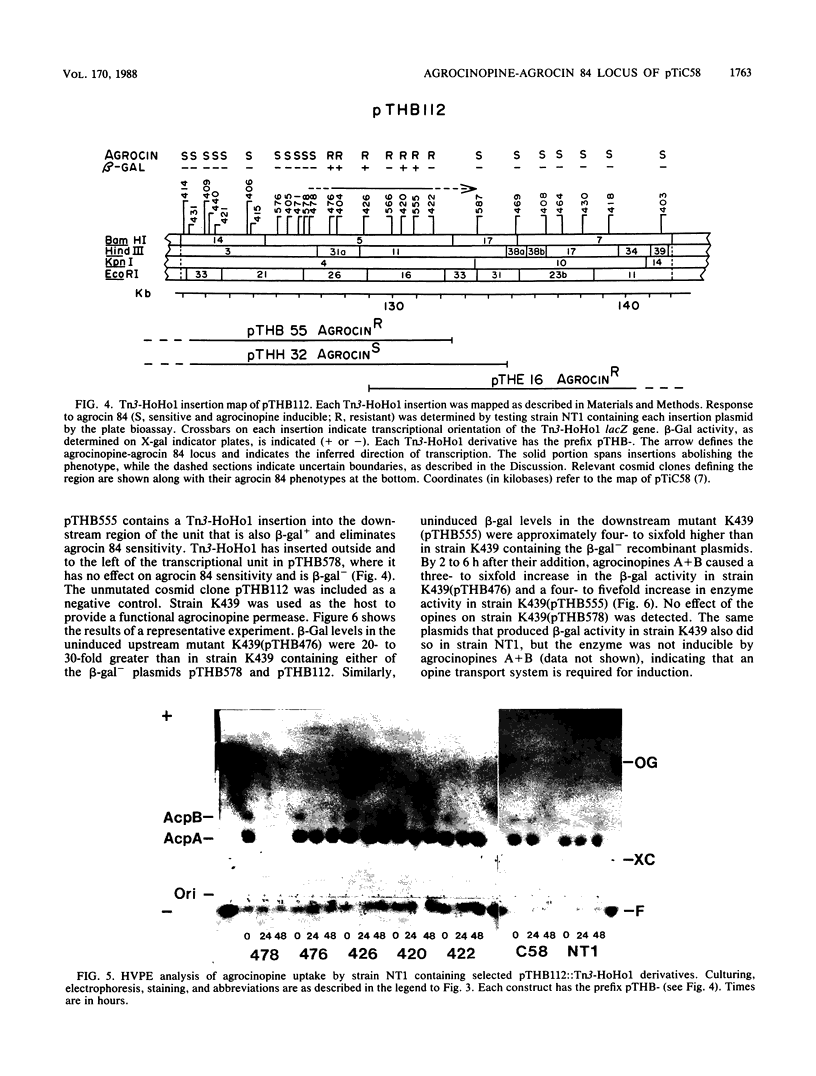
Overlapping segments of pTiC58 inserted into cosmid vectors were used to characterize the agrocinopine-agrocin 84 locus from the nopaline/agrocinopine A and B Agrobacterium tumefaciens strain C58. All of the clones conferring agrocin 84 sensitivity on agrobacteria also conferred uptake of agrocin 84 and agrocinopines A and B. Transposon Tn3-HoHo1 insertion mutations of one such clone were generated that simultaneously abolished agrocin 84 sensitivity and transport of agrocinopines A and B and agrocin 84. Such insertions were found to cluster within a 4.4-kilobase region. Analysis of beta-galactosidase activity in these insertion mutants suggested a single transcriptional unit regulated at the transcriptional level by agrocinopines A and B. The smallest DNA fragment subcloned from the region to confer all three activities was 8.5 kilobases long. This subclone was still properly regulated, indicating that the regulatory gene is closely linked to the locus. The data are consistent with a single operon encoding catabolism of agrocinopines A and B and conferring sensitivity to agrocin 84. Based on these results, we support the locus name acc, for agrocinopine catabolism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Currier T. C., Farrand S. K., Bendich A. J., Gordon M. P., Nester E. W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey D. A. A spontaneous insertion in the agrocin sensitivity region of the Ti-plasmid of Agrobacterium tumefaciens C58. Plasmid. 1986 Nov;16(3):222–224. doi: 10.1016/0147-619x(86)90061-2. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new color reaction for the determination of aldopentose in presence of other saccharides. Biochim Biophys Acta. 1957 Mar;23(3):639–642. doi: 10.1016/0006-3002(57)90387-6. [DOI] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depicker A., De Wilde M., De Vos G., De Vos R., Van Montagu M., Schell J. Molecular cloning of overlapping segments of the nopaline Ti-plasmid pTiC58 as a means to restriction endonuclease mapping. Plasmid. 1980 Mar;3(2):193–211. doi: 10.1016/0147-619x(80)90109-2. [DOI] [PubMed] [Google Scholar]
- Dessaux Y., Guyon P., Farrand S. K., Petit A., Tempé J. Agrobacterium Ti and Ri plasmids specify enzymic lactonization of mannopine to agropine. J Gen Microbiol. 1986 Sep;132(9):2549–2559. doi: 10.1099/00221287-132-9-2549. [DOI] [PubMed] [Google Scholar]
- Dessaux Y., Tempé J., Farrand S. K. Genetic analysis of mannityl opine catabolism in octopine-type Agrobacterium tumefaciens strain 15955. Mol Gen Genet. 1987 Jun;208(1-2):301–308. doi: 10.1007/BF00330457. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S. K., Slota J. E., Shim J. S., Kerr A. Tn5 insertions in the agrocin 84 plasmid: the conjugal nature of pAgK84 and the locations of determinants for transfer and agrocin 84 production. Plasmid. 1985 Mar;13(2):106–117. doi: 10.1016/0147-619x(85)90063-0. [DOI] [PubMed] [Google Scholar]
- Hirooka T., Kado C. I. Location of the right boundary of the virulence region on Agrobacterium tumefaciens plasmid pTiC58 and a host-specifying gene next to the boundary. J Bacteriol. 1986 Oct;168(1):237–243. doi: 10.1128/jb.168.1.237-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Holsters M., de Waele D., Depicker A., Messens E., van Montagu M., Schell J. Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet. 1978 Jul 11;163(2):181–187. doi: 10.1007/BF00267408. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Messens E., Lenaerts A., Hedges R. W., Van Montagu M. Agrocinopine A, a phosphorylated opine is secreted from crown gall cells. EMBO J. 1985 Mar;4(3):571–577. doi: 10.1002/j.1460-2075.1985.tb03668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder M. H., Tate M. E., Jones G. P. Agrocinopine A, a tumor-inducing plasmid-coded enzyme product, is a phosphodiester of sucrose and L-arabinose. J Biol Chem. 1984 Aug 10;259(15):9704–9710. [PubMed] [Google Scholar]
- STONIER T. Agrobacterium tumefaciens Conn. II. Production of an antibiotic substance. J Bacteriol. 1960 Jun;79:889–898. doi: 10.1128/jb.79.6.889-898.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slota J. E., Farrand S. K. Genetic isolation and physical characterization of pAgK84, the plasmid responsible for agrocin 84 production. Plasmid. 1982 Sep;8(2):175–186. doi: 10.1016/0147-619x(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tempé J., Petit A., Holsters M., Montagu M., Schell J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: Possible relation to transformation in crown gall. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky M. F., Nester E. W. Molecular characterization of a host-range-determining locus from Agrobacterium tumefaciens. J Bacteriol. 1986 Oct;168(1):244–250. doi: 10.1128/jb.168.1.244-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerer R. P., Hamilton R. H., Pootjes C. Isolation and morphology of temperature Agrobacterium tumefaciens bacteriophage. J Bacteriol. 1966 Sep;92(3):746–750. doi: 10.1128/jb.92.3.746-750.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]