Abstract
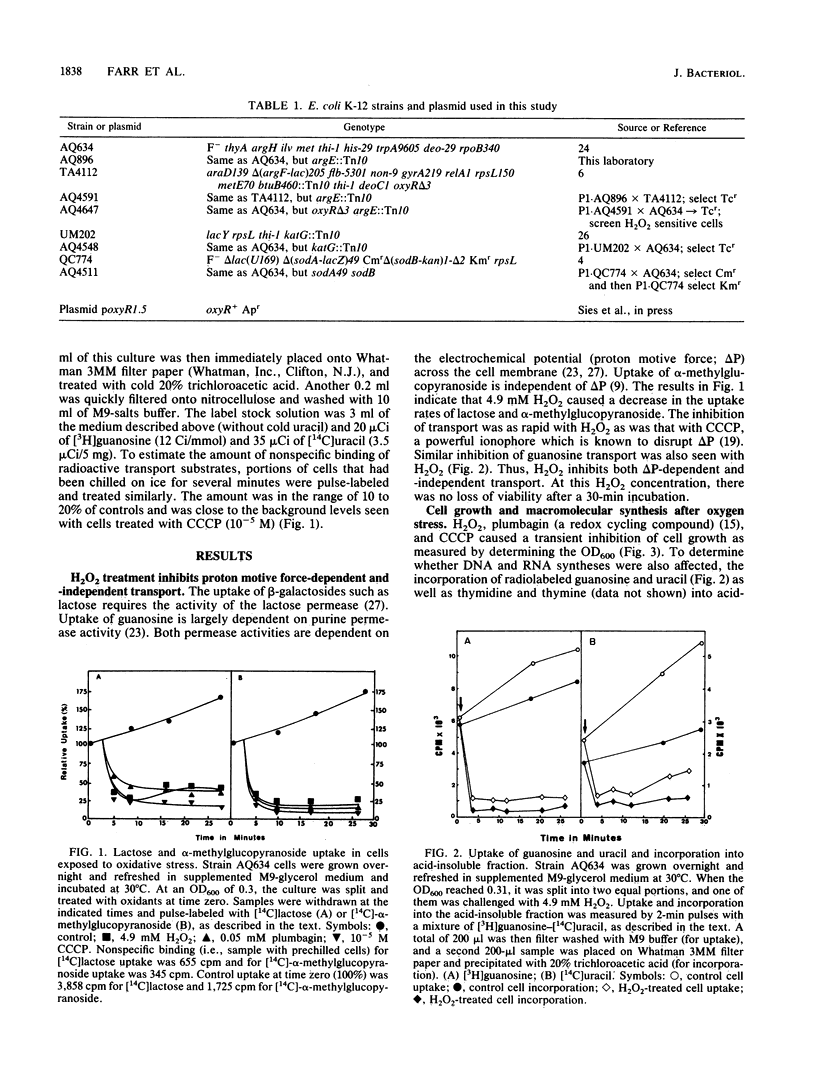
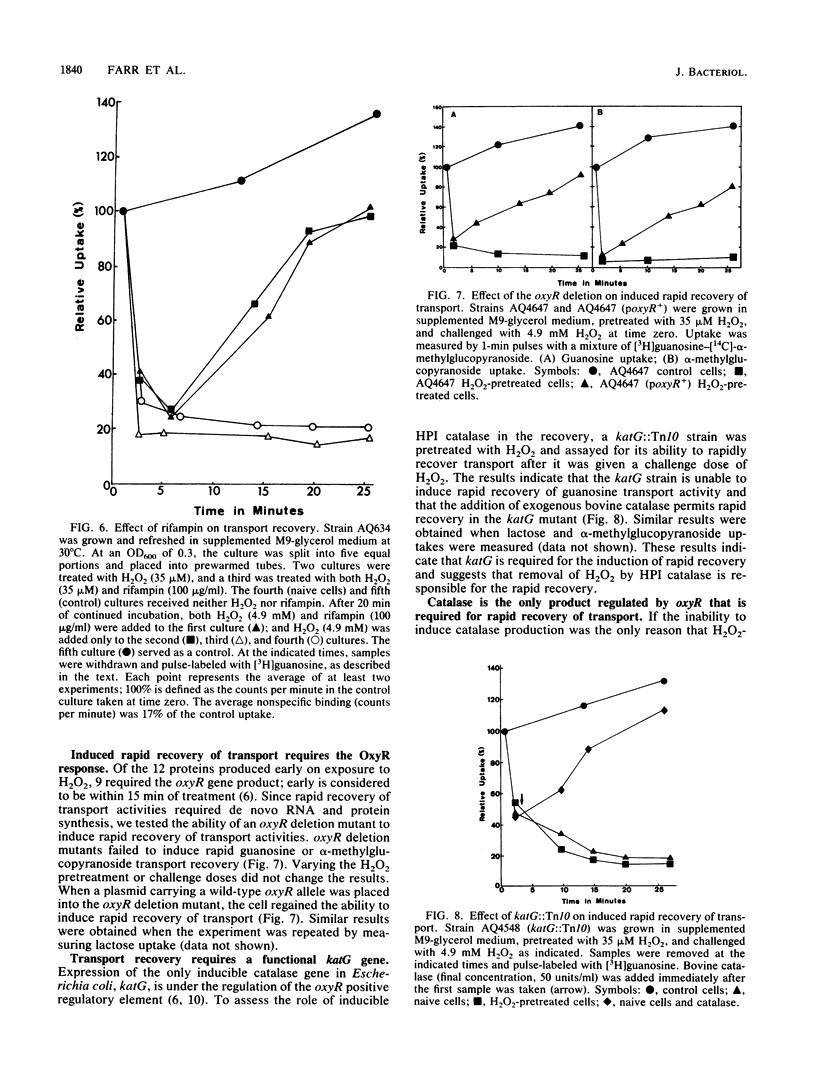
Different conditions of oxidative stress were used to study their effects on membrane transport in Escherichia coli K-12. The oxidizing conditions included H2O2, plumbagin (a redox cycling compound that generates superoxide radicals [O2-]), and increased partial pressure of oxygen. Both superoxide radical-generating conditions and H2O2 treatments were found to cause a rapid decrease in proton motive force-dependent and -independent transport. H2O2-pretreated cells had the ability to rapidly recover both proton motive force-dependent and -independent transport. The induction required transcription and translation and was dependent on oxyR+ and katG+, providing evidence that these genes play crucial roles in the rapid recovery of transport. The effects of oxidatively induced loss of proton motive force on cell growth and macromolecular synthesis were also investigated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boehm D. E., Vincent K., Brown O. R. Oxygen and toxicity inhibition of amino acid biosynthesis. Nature. 1976 Jul 29;262(5567):418–420. doi: 10.1038/262418a0. [DOI] [PubMed] [Google Scholar]
- Brown O. R., Seither R. L. Oxygen and redox-active drugs: shared toxicity sites. Fundam Appl Toxicol. 1983 Jul-Aug;3(4):209–214. doi: 10.1016/s0272-0590(83)80127-4. [DOI] [PubMed] [Google Scholar]
- Brunori M., Rotilio G. Biochemistry of oxygen radical species. Methods Enzymol. 1984;105:22–35. doi: 10.1016/s0076-6879(84)05005-9. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E., Weiss B. Endonuclease IV of Escherichia coli is induced by paraquat. Proc Natl Acad Sci U S A. 1987 May;84(10):3189–3193. doi: 10.1073/pnas.84.10.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstark A., Perrot G. Catalase has only a minor role in protection against near-ultraviolet radiation damage in bacteria. Mol Gen Genet. 1987 Apr;207(1):68–72. doi: 10.1007/BF00331492. [DOI] [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Natvig D. O., Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1309–1316. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases: regularities and irregularities. Harvey Lect. 1983 1984;79:51–75. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol. 1987 Jul;169(7):2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- Kramer G. F., Ames B. N. Oxidative mechanisms of toxicity of low-intensity near-UV light in Salmonella typhimurium. J Bacteriol. 1987 May;169(5):2259–2266. doi: 10.1128/jb.169.5.2259-2266.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A., Mygind B., Nicolaisen A., Pihl N. J. Nucleoside transport in cells and membrane vesicles from Escherichia coli K12. J Biol Chem. 1979 May 25;254(10):3730–3737. [PubMed] [Google Scholar]
- Ogawa T., Pickett G. G., Kogoma T., Kornberg A. RNase H confers specificity in the dnaA-dependent initiation of replication at the unique origin of the Escherichia coli chromosome in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1040–1044. doi: 10.1073/pnas.81.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seither R. L., Brown O. R. Induction of stringency by hyperoxia in Escherichia coli. Cell Mol Biol. 1982;28(3):285–291. [PubMed] [Google Scholar]
- Triggs-Raine B. L., Loewen P. C. Physical characterization of katG, encoding catalase HPI of Escherichia coli. Gene. 1987;52(2-3):121–128. doi: 10.1016/0378-1119(87)90038-2. [DOI] [PubMed] [Google Scholar]
- West I. C. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970 Nov 9;41(3):655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- Wilson D. M., Alderette J. F., Maloney P. C., Wilson T. H. Protonmotive force as the source of energy for adenosine 5'-triphosphate synthesis in Escherichia coli. J Bacteriol. 1976 Apr;126(1):327–337. doi: 10.1128/jb.126.1.327-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


