Abstract
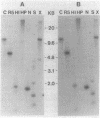
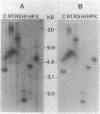
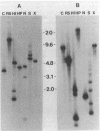
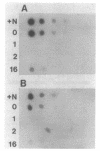
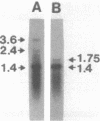
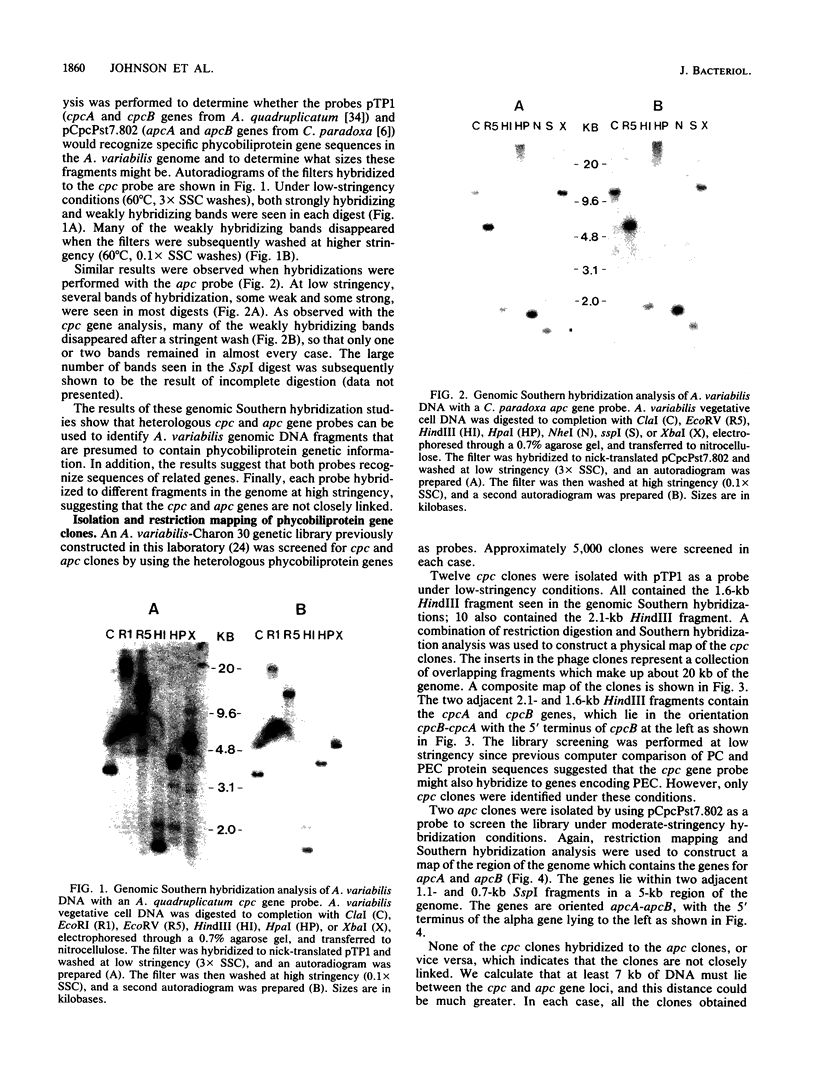
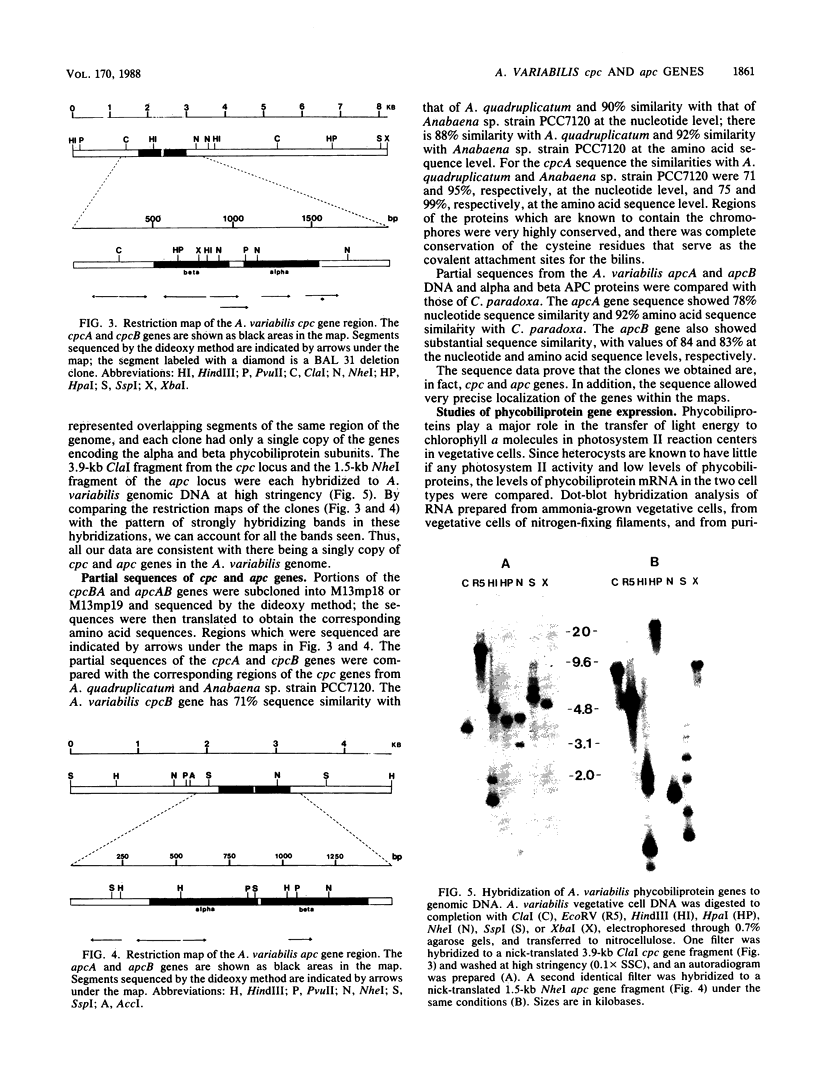
Gene clones encoding phycocyanin and allophycocyanin were isolated from an Anabaena variabilis ATCC 29413-Charon 30 library by using the phycocyanin (cpc) genes of Agmenellum quadruplicatum and the allophycocyanin (apc) genes of Cyanophora paradoxa as heterologous probes. The A. variabilis cpcA and cpcB genes occur together in the genome, as do the apcA and apcB genes; the two sets of genes are not closely linked, however. The cpc and apc genes appear to be present in only one copy per genome. DNA-RNA hybridization analysis showed that expression of the cpc and apc genes is greatly decreased during nitrogen starvation; within 1 h no cpc or apc mRNA could be detected. The source of nitrogen for growth did not influence expression of the genes; vegetative cells from nitrogen-fixing and ammonia-grown cultures had approximately the same levels of cpc and apc mRNAs. Heterocysts had less than 5% as much cpc mRNA as vegetative cells from nitrogen-fixing cultures. Northern hybridization (RNA blot) analysis showed that the cpc genes are transcribed to give an abundant 1.4-kilobase (kb) RNA as well as two less prominent 3.8- and 2.6-kb species. The apc genes gave rise to two transcripts, a 1.4-kb predominant RNA and a minor 1.75-kb form.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Anderson L. K., Rayner M. C., Sweet R. M., Eiserling F. A. Regulation of Nostoc sp. phycobilisome structure by light and temperature. J Bacteriol. 1983 Sep;155(3):1407–1416. doi: 10.1128/jb.155.3.1407-1416.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap W. R., Haselkorn R. Cloning and light regulation of expression of the phycocyanin operon of the cyanobacterium Anabaena. EMBO J. 1987 Apr;6(4):871–884. doi: 10.1002/j.1460-2075.1987.tb04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. A., Glazer A. N., Eiserling F. A. Characterization and structural properties of the major biliproteins of Anabaena sp. Arch Microbiol. 1976 Oct 11;110(1):61–75. doi: 10.1007/BF00416970. [DOI] [PubMed] [Google Scholar]
- Bryant D. A., de Lorimier R., Lambert D. H., Dubbs J. M., Stirewalt V. L., Stevens S. E., Jr, Porter R. D., Tam J., Jay E. Molecular cloning and nucleotide sequence of the alpha and beta subunits of allophycocyanin from the cyanelle genome of Cyanophora paradoxa. Proc Natl Acad Sci U S A. 1985 May;82(10):3242–3246. doi: 10.1073/pnas.82.10.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Lomax T. L., Grossman A. R. Genes encoding major light-harvesting polypeptides are clustered on the genome of the cyanobacterium Fremyella diplosiphon. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3924–3928. doi: 10.1073/pnas.83.11.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Clark J. H. Phycobilisomes: macromolecular structure and energy flow dynamics. Biophys J. 1986 Jan;49(1):115–116. doi: 10.1016/S0006-3495(86)83616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. R., Lemaux P. G., Conley P. B. Regulated synthesis of phycobilisome components. Photochem Photobiol. 1986 Dec;44(6):827–837. doi: 10.1111/j.1751-1097.1986.tb05543.x. [DOI] [PubMed] [Google Scholar]
- Hardie L. P., Balkwill D. L., Stevens S. E. Effects of Iron Starvation on the Physiology of the Cyanobacterium Agmenellum quadruplicatum. Appl Environ Microbiol. 1983 Mar;45(3):999–1006. doi: 10.1128/aem.45.3.999-1006.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helber J. T., Johnson T. R., Yarbrough L. R., Hirschberg R. Effect of nitrogenous compounds on nitrogenase gene expression in anaerobic cultures of Anabaena variabilis. J Bacteriol. 1988 Feb;170(2):558–563. doi: 10.1128/jb.170.2.558-563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helber J. T., Johnson T. R., Yarbrough L. R., Hirschberg R. Regulation of nitrogenase gene expression in anaerobic cultures of Anabaena variabilis. J Bacteriol. 1988 Feb;170(2):552–557. doi: 10.1128/jb.170.2.552-557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A., Wolk C. P. Genetic mapping of the chromosome of the cyanobacterium, Anabaena variabilis. Proximity of the structural genes for nitrogenase and ribulose-bisphosphate carboxylase. J Biol Chem. 1986 Jun 15;261(17):7748–7754. [PubMed] [Google Scholar]
- Houmard J., Mazel D., Moguet C., Bryant D. A., Tandeau de Marsac N. Organization and nucleotide sequence of genes encoding core components of the phycobilisomes from Synechococcus 6301. Mol Gen Genet. 1986 Dec;205(3):404–410. doi: 10.1007/BF00338074. [DOI] [PubMed] [Google Scholar]
- Lambert D. H., Bryant D. A., Stirewalt V. L., Dubbs J. M., Stevens S. E., Jr, Porter R. D. Gene map for the Cyanophora paradoxa cyanelle genome. J Bacteriol. 1985 Nov;164(2):659–664. doi: 10.1128/jb.164.2.659-664.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau R. H., MacKenzie M. M., Doolittle W. F. Phycocyanin synthesis and degradation in the blue-green bacterium Anacystis nidulans. J Bacteriol. 1977 Dec;132(3):771–778. doi: 10.1128/jb.132.3.771-778.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaux P. G., Grossman A. R. Major light-harvesting polypeptides encoded in polycistronic transcripts in a eukaryotic alga. EMBO J. 1985 Aug;4(8):1911–1919. doi: 10.1002/j.1460-2075.1985.tb03870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Rice D., Haselkorn R. Identification of blue-green algal nitrogen fixation genes by using heterologous DNA hybridization probes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):186–190. doi: 10.1073/pnas.77.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Dolan E., Calvert H. E., Ke B. Energy transfer from phycobiliproteins to photosystem I in vegetative cells and heterocysts of Anabaena variabilis. Biochim Biophys Acta. 1981 Feb 12;634(2):237–248. doi: 10.1016/0005-2728(81)90142-0. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Wolk C. P. High recovery of nitrogenase activity and of Fe-labeled nitrogenase in heterocysts isolated from Anabaena variabilis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6271–6275. doi: 10.1073/pnas.75.12.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot T. J., Fox J. L. Cloning and sequencing of the genes encoding the alpha and beta subunits of C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6983–6987. doi: 10.1073/pnas.81.22.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. E., Paone D. A. Accumulation of Cyanophycin Granules as a Result of Phosphate Limitation in Agmenellum quadruplicatum. Plant Physiol. 1981 Apr;67(4):716–719. doi: 10.1104/pp.67.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N. B., Haselkorn R. Control of phycobiliprotein proteolysis and heterocyst differentiation in Anabaena. J Bacteriol. 1980 Mar;141(3):1375–1385. doi: 10.1128/jb.141.3.1375-1385.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Kiley P. J., Donohue T. J., Kaplan S. Origin of the mRNA stoichiometry of the puf operon in Rhodobacter sphaeroides. J Biol Chem. 1986 Aug 5;261(22):10366–10374. [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]