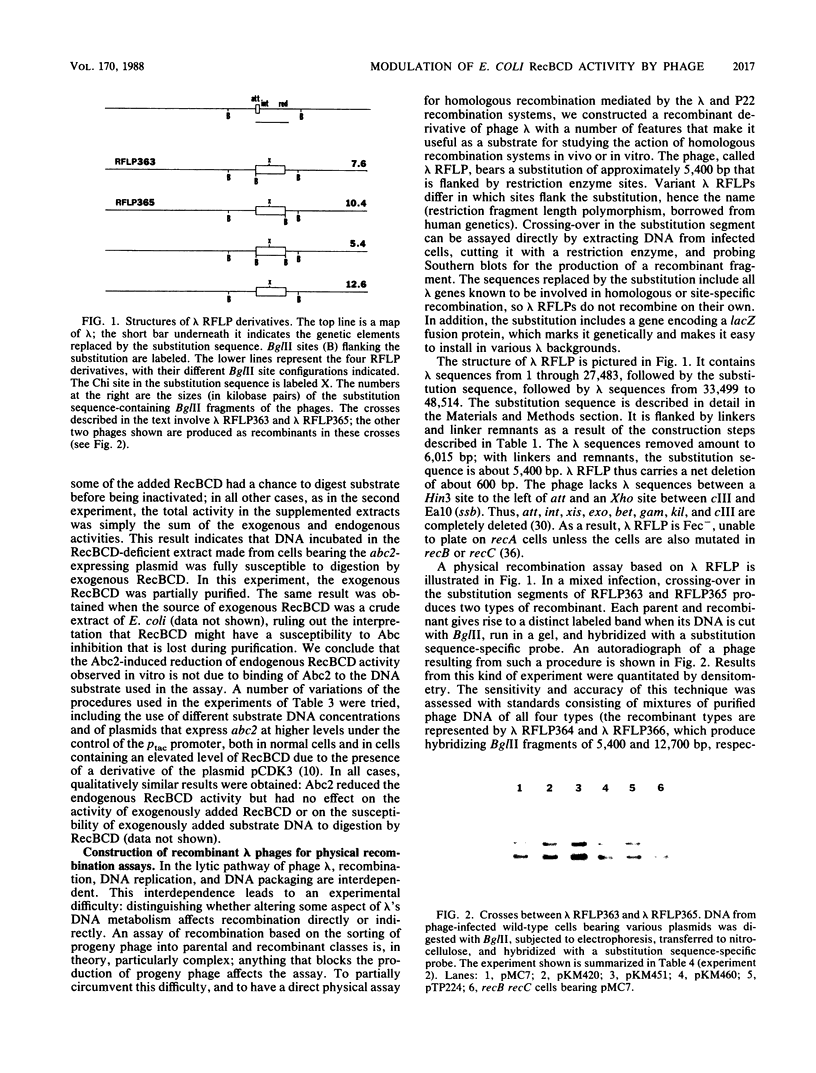
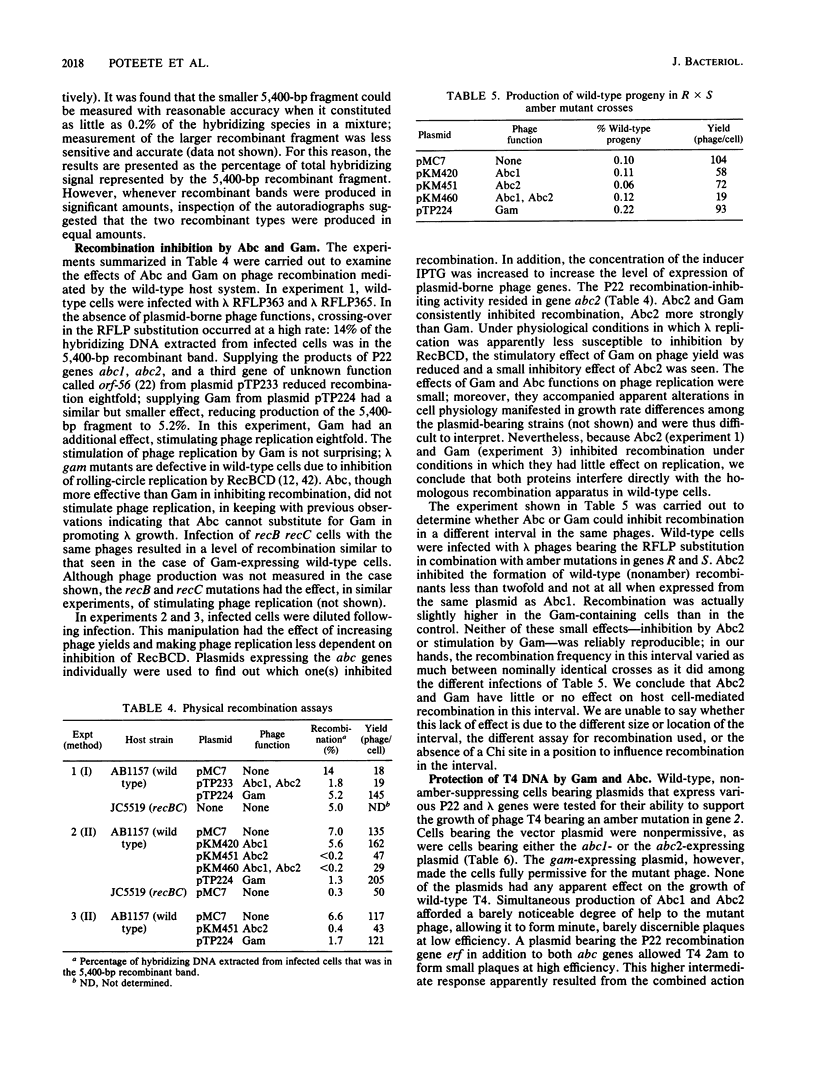
Abstract
Plasmids that express the bacteriophage lambda gam gene or the P22 abc2 gene (with and without abc1) at controllable levels were placed in Escherichia coli and tested for effects on the activity of RecBCD. Like Gam, Abc2 inhibited the ATP-dependent exonuclease activity of RecBCD, apparently not by binding to DNA. However, Abc2-mediated inhibition was partial, while Gam-mediated inhibition was complete. Both Abc2 and Gam inhibited host system-mediated homologous recombination in a Chi-containing interval in the chromosome of a hybrid lambda phage; Abc2 inhibited it more strongly than Gam. Gam but not Abc2 spared a phage T4 gene 2 mutant from restriction by RecBCD; Abc2 exhibited weak sparing activity in combination with Abc1 and substantial activity in combination with both Abc1 and P22 homologous recombination function Erf. Either Gam or the combination of the lambda recombination functions Exo and Bet was sufficient to induce a mode of plasmid replication that produced linear multimers. The combination of Abc2, Abc1, and Erf also exhibited this activity. However, Erf was inactive, both by itself and in combination with Abc1; Abc2 had weak activity. These results indicate that Gam and Abc2 modulate the activity of RecBCD in significantly different ways. In comparison with lambda Gam, P22 Abc2 has a weak effect on RecBCD nuclease activity but a strong effect on its recombination-promoting activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amundsen S. K., Taylor A. F., Chaudhury A. M., Smith G. R. recD: the gene for an essential third subunit of exonuclease V. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5558–5562. doi: 10.1073/pnas.83.15.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behme M. T., Lilley G. D., Ebisuzaki K. Postinfection control by bacteriophage T4 of Escherichia coli recBC nuclease activity. J Virol. 1976 Apr;18(1):20–25. doi: 10.1128/jvi.18.1.20-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget P. B., Poteete A. R., Sauer R. T. Control of phage P22 tail protein expression by transcription termination. J Mol Biol. 1983 Mar 15;164(4):561–572. doi: 10.1016/0022-2836(83)90050-5. [DOI] [PubMed] [Google Scholar]
- Biek D. P., Cohen S. N. Identification and characterization of recD, a gene affecting plasmid maintenance and recombination in Escherichia coli. J Bacteriol. 1986 Aug;167(2):594–603. doi: 10.1128/jb.167.2.594-603.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen A., Clark A. J. Synthesis of linear plasmid multimers in Escherichia coli K-12. J Bacteriol. 1986 Jul;167(1):327–335. doi: 10.1128/jb.167.1.327-335.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra C. C., Prasher D., Kushner S. R. Physical and biochemical analysis of the cloned recB and recC genes of Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):21–27. doi: 10.1128/jb.157.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler D. C., Lehman I. R. On the role of ATP in phosphodiester bond hydrolysis catalyzed by the recBC deoxyribonuclease of Escherichia coli. J Biol Chem. 1977 Jan 25;252(2):499–503. [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Fenton A. C., Poteete A. R. Genetic analysis of the erf region of the bacteriophage P22 chromosome. Virology. 1984 Apr 15;134(1):148–160. doi: 10.1016/0042-6822(84)90280-0. [DOI] [PubMed] [Google Scholar]
- Friedman S. A., Hays J. B. Selective inhibition of Escherichia coli recBC activities by plasmid-encoded GamS function of phage lambda. Gene. 1986;43(3):255–263. doi: 10.1016/0378-1119(86)90214-3. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Smith T. A., Friedman S. A., Lee E., Coffman G. L. RecF and RecBC function during recombination of nonreplicating, UV-irradiated phage lambda DNA and during other recombination processes. Cold Spring Harb Symp Quant Biol. 1984;49:475–483. doi: 10.1101/sqb.1984.049.01.054. [DOI] [PubMed] [Google Scholar]
- Hilliker S., Botstein D. Specificity of genetic elements controlling regulation of early functions in temperate bacteriophages. J Mol Biol. 1976 Sep 25;106(3):537–566. doi: 10.1016/0022-2836(76)90251-5. [DOI] [PubMed] [Google Scholar]
- Karu A. E., Sakaki Y., Echols H., Linn S. The gamma protein specified by bacteriophage gamma. Structure and inhibitory activity for the recBC enzyme of Escherichia coli. J Biol Chem. 1975 Sep 25;250(18):7377–7387. [PubMed] [Google Scholar]
- Liao S. M., Wu T. H., Chiang C. H., Susskind M. M., McClure W. R. Control of gene expression in bacteriophage P22 by a small antisense RNA. I. Characterization in vitro of the Psar promoter and the sar RNA transcript. Genes Dev. 1987 Apr;1(2):197–203. doi: 10.1101/gad.1.2.197. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Sironi G., Bialy H., Calendar R. Bacteriophage lambda; abortive infection of bacteria lysogenic for phage P2. Proc Natl Acad Sci U S A. 1970 Jul;66(3):587–594. doi: 10.1073/pnas.66.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. C., Fenton A. C., Poteete A. R. Sequence of the bacteriophage P22 anti-recBCD (abc) genes and properties of P22 abc region deletion mutants. Virology. 1987 Oct;160(2):456–464. doi: 10.1016/0042-6822(87)90017-1. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Goldberg E. B. Protection of parental T4 DNA from a restriction exonuclease by the product of gene 2. J Mol Biol. 1977 Nov;116(4):877–881. doi: 10.1016/0022-2836(77)90276-5. [DOI] [PubMed] [Google Scholar]
- Pacumbaba R., Center M. S. Partial purification and properties of a bacteriophage T7 inhibitor of the host exonuclease V activity. J Virol. 1975 Nov;16(5):1200–1207. doi: 10.1128/jvi.16.5.1200-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteete A. R., Fenton A. C. DNA-binding properties of the Erf protein of bacteriophage P22. J Mol Biol. 1983 Jan 15;163(2):257–275. doi: 10.1016/0022-2836(83)90006-2. [DOI] [PubMed] [Google Scholar]
- Poteete A. R., Fenton A. C. Lambda red-dependent growth and recombination of phage P22. Virology. 1984 Apr 15;134(1):161–167. doi: 10.1016/0042-6822(84)90281-2. [DOI] [PubMed] [Google Scholar]
- Poteete A. R. Location and sequence of the erf gene of phage P22. Virology. 1982 Jun;119(2):422–429. doi: 10.1016/0042-6822(82)90101-5. [DOI] [PubMed] [Google Scholar]
- Sakaki Y. Inactivation of the ATP-dependent DNase of Escherichia coli after infection with double-stranded DNA phages. J Virol. 1974 Dec;14(6):1611–1612. doi: 10.1128/jvi.14.6.1611-1612.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Krovatin W., DeAnda J., Youderian P., Susskind M. M. Primary structure of the immI immunity region of bacteriophage P22. J Mol Biol. 1983 Aug 25;168(4):699–713. doi: 10.1016/s0022-2836(83)80070-9. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Krovatin W., Poteete A. R., Berget P. B. Phage P22 tail protein: gene and amino acid sequence. Biochemistry. 1982 Nov 9;21(23):5811–5815. doi: 10.1021/bi00266a014. [DOI] [PubMed] [Google Scholar]
- Sironi G. Mutants of Escherichia coli unable to be lysogenized by the temperate bacteriophage P2. Virology. 1969 Feb;37(2):163–176. doi: 10.1016/0042-6822(69)90196-2. [DOI] [PubMed] [Google Scholar]
- Skalka A. M. DNA replication--bacteriophage lambda. Curr Top Microbiol Immunol. 1977;78:201–237. [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Unger R. C., Clark A. J. Interaction of the recombination pathways of bacteriophage lambda and its host Escherichia coli K12: effects on exonuclease V activity. J Mol Biol. 1972 Oct 14;70(3):539–548. doi: 10.1016/0022-2836(72)90558-x. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Radding C. M. Partial purification and properties of an exonuclease inhibitor induced by bacteriophage Mu-1. J Virol. 1981 Aug;39(2):548–558. doi: 10.1128/jvi.39.2.548-558.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Botstein D. Control of lysogenization by phage P22. I. The P22 cro gene. J Mol Biol. 1981 Oct 25;152(2):209–232. doi: 10.1016/0022-2836(81)90240-0. [DOI] [PubMed] [Google Scholar]
- Zagursky R. J., Hays J. B. Expression of the phage lambda recombination genes exo and bet under lacPO control on a multi-copy plasmid. Gene. 1983 Sep;23(3):277–292. doi: 10.1016/0378-1119(83)90018-5. [DOI] [PubMed] [Google Scholar]