Abstract
Human thioredoxin reductase (TR) contains selenocysteine (Secys) in a redox center [cysteine (Cys)-497,Secys-498] near the C-terminus. The essential role of Secys in TR isolated from HeLa cells was demonstrated by the alkylation studies. Reaction of native NADPH reduced enzyme with bromoacetate at pH 6.5 inhibited enzyme activity 99%. Of the incorporated carboxymethyl (CM) group, 1.1 per subunit, >90% was in CM-Secys-498. Alkylation at pH 8 increased the stoichiometry to 1.6 per subunit with additional modification of the Cys-59, Cys-64 disulfide center. A minor tryptic peptide containing both CM-Cys-497 and CM-Secys-498 was isolated from enzyme alkylated at pH 6.5 or at pH 8. Preparations of TR isolated from HeLa cells grown in a fermentor under high aeration contained selenium-deficient enzyme species that had 50% lower activity. Decreasing oxygen to an optimal level increased cell yield, and fully active TR containing one Se per subunit was present. Reduction of fully active enzyme with tris-(2-carboxyethyl) phosphine converted it from a low to a high heparin affinity form. The tris-(2-carboxyethyl) phosphine-reduced enzyme was oxygen-sensitive and lost selenium and catalytic activity unless maintained under strictly anaerobic conditions. This enzyme could be converted to an oxygen-insensitive species by addition of NADPH, indicating that bound pyridine nucleotide is important for enzyme stability. An induced enzyme conformation in which the essential Secys is shielded from oxidative damage could explain these effects.
Keywords: selenoprotein/redox active centers/transformed cell lines/bromoacetate
Thioredoxin reductase (TR) is an NADPH-dependent, FAD-containing disulfide reductase that plays an important role in cell proliferation (1). Unlike the well characterized homologues from yeast and prokaryotes, the larger mammalian enzyme is a selenoprotein that contains a selenocysteine (Secys) residue (2) in the sequence -Cys-Secys-Gly (end) at the C terminus of each subunit (3, 4, 5). Catalysis of electron transfer from NADPH to thioredoxin, which in turn is linked to critical components of cell metabolism such as ribonucleotide reductase (6), AP-1 and NF-κB transcription factors (7–10), vitamin K epoxide reductase (11), thiolperoxidase (12), and plasma glutathione peroxidase (13), illustrates the diversity of processes that depend on this selenium-containing TR. The provision of reduced thioredoxin for two very important cell processes, DNA synthesis and gene transcription, implicates TR as a key enzyme in the control of cell growth. The increased amount of TR in mammalian tumor cells compared with normal tissue (4) suggests a role of this enzyme in malignancy development.
Reported catalytic activities of mammalian TRs with disulfide substrates such as homologous thioredoxin, Escherichia coli thioredoxin, or the artificial disulfide 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) differ considerably depending on the source of TR and the isolation procedure used. Before the identification of the enzyme as a selenoprotein, inadequate precautions to avoid oxidative damage during isolation may have been a factor. Reported variations in enzyme stability, pI values varying from 4.8 (14) to 5.8 (15) for TR from different sources, and observations that in a single purified preparation two forms with distinct pI values of 5.5 and 5.8 (15) or 5.15 and 5.35 (3) occur are further indications of the heterogeneity of highly purified mammalian TR preparations. More recent studies showing dependency of TR activity in cultured cell lines on availability of selenium in growth media (16, 17) indicated an essential role of selenocysteine in the enzyme. As shown (4), TR isolated from HeLa cells and from human lung adenocarcinoma cells contained two major enzyme species separable by heparin–agarose affinity chromatography. The low-affinity enzyme form had strong reactivity with anti-rat liver TR polyclonal antibodies whereas the high-affinity form was not detected by immunoblot assay. In the present study, two forms of TR were isolated from HeLa cells by heparin affinity chromatography and were characterized. Low catalytic activity of one of these forms was correlated with a low selenium content. Reduction of enzyme activity to 1% or less concomitant with selective alkylation of the Secys-498 residue further indicates the essential role of selenocysteine in catalysis. In this case, reaction of native NADPH reduced enzyme with bromoacetate not only inhibited enzyme activity by 99% but also resulted in incorporation of 1.1 equivalents of alkyl group, of which >90% was present in the carboxymethyl (CM)-derivative of Secys-498 and ≈5% was present in the CM-derivative of Cys-497. Clearly, the susceptibility of this C-terminal redox center to alkylation at pH 6.5, which is near to the optimum pH range for reduction of a disulfide substrate, points to the accessibility of the reactive ionized selenol group under these conditions and its importance for catalytic activity.
MATERIALS AND METHODS
Materials.
[75Se] selenious acid (30.5 Ci/mmol Se) was from the Research Reactor Facility (University of Missouri, Columbia) or from Los Alamos National Laboratory (Los Alamos, NM). Bromo[1-14C]acetic acid (57 mCi/mmol) was from American Radiolabeled Chemicals (St. Louis). Heat-inactivated fetal bovine serum and DMEM medium were from Irvine Scientific. E. coli recombinant thioredoxin and Tris-(2-carboxyethyl)phosphine (TCEP) were from Calbiochem. Glutathione, N-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin, S-carboxymethyl-l-cysteine and seleno-dl-cystine were from Sigma. A Supelco heparin-5PW Progel-TSK HPLC column, 7.5 cm × 7.5 mm, was from Thomson Instruments (Springfield, VA). 2′,5′-ADP Sepharose-4B, phenyl-Sepharose CL-4B, and DEAE Sepharose Fast Flow were from Pharmacia LKB. A C18 reversed-phase HPLC column was from Vydac (Hesperia, CA). Precast polyacrylamide gels and molecular weight standards were from NOVEX (San Diego).
Cell Growth.
HeLa cells were grown in 7 liters of DMEM supplemented with 10% heat-inactivated fetal bovine serum and 1% antibiotic–antimycotic solution in a 14-liter fermentor, BIOFLO-3000. Cultivation parameters were maintained throughout growth at 35°C, pH 7.2, and dissolved oxygen saturated at 50% or 30% according to the manufacturer’s specifications. For 75Se-labeled cells, each batch culture was supplemented with 0.4 μM sodium selenite and 1 mCi of [75Se]selenite. For production of nonradioactive cells, media contained 1 μM sodium selenite. Cells were harvested after 4 days at a density of 1–2 × 106 cells/ml, were washed with 150 mM NaCl in 10 mM potassium phosphate buffer (pH 7.0), and were frozen at −80°C until used.
TR Purification.
Frozen 75Se-labeled cells or a mixture of labeled and unlabeled frozen cells were thawed in 20 mM potassium phosphate (pH 7.0), 1 mM EDTA (buffer A) supplemented with 1 mM DTT, 0.1% phenylmethylsulfonyl fluoride, and crystals of DNase I. Cells were disrupted by sonication. After centrifugation, the supernatant was loaded on a DEAE-Sepharose Fast Flow column equilibrated with buffer A containing 1 mM DTT, and, after washing with two volumes of the same buffer, a linear gradient of 0–1M KCl in the buffer was applied. TR was purified further from fractions containing radioactivity by phenyl-Sepharose CL-4B chromatography followed by affinity chromatography on 2′,5′-ADP Sepharose-4B as described elsewhere (3). The enzyme preparation was ≈95% pure after these steps. After dilution and concentration, the enzyme solution was applied to a heparin HPLC column for further purification and separation of the enzyme forms. Buffer A was used throughout all of these steps of purification.
When the enzyme forms were purified under anaerobic conditions, buffers purged with helium were used to elute protein from the heparin HPLC column. Fractions were collected in glass tubes under argon and were hermetically sealed with a rubber seal. Centricon-30 concentrators placed in 50-ml argon-filled glass centrifuge tubes containing 2 ml of 0.5 M sodium dithionate and benzylviologen, as redox indicator, were used for enzyme concentration.
Protein Assay.
Protein content was determined by Bio-Rad dye reagent or modified Lowry method (Pierce). BSA was used as standard.
Spectroscopic Properties.
The absorption spectra were recorded on a U-2000 spectrophotometer (Hitachi, Tokyo). Enzyme samples (≈0.3 mg of protein per ml) were prepared in buffer A with 10% glycerol.
TR Assay.
The assay mixture (0.5 ml) contained 100 mM potassium phosphate (pH 7.0) 10 mM EDTA, 0.2 mM NADPH, and 2.5 mM DTNB. In the alternative assay, 40 μM E. coli recombinant thioredoxin (oxidized form) and 80 μM insulin or 2 mM oxidized glutathione were used instead of DTNB. The reaction was started by the addition of the enzyme, and the increase in absorbance at 412 nm with DTNB or the rate of NADPH oxidation in the presence of the other substrates was monitored at 30°C. The rate of DTNB reduction as a function of pH was measured in 100 mM potassium phosphate buffers prepared from equimolar K2HPO4 and KH2PO4.
Carboxymethylation of TR.
The 75Se-labeled low heparin affinity enzyme form (11.0 nmol; 5.8 × 105 cpm) in 200 μl of 20 mM potassium phosphate (pH 7.0), 1 mM EDTA, and 10% glycerol was purged with argon and was mixed with 25 μl of 1 M potassium phosphate (pH 6.5) and 5 μl of NADPH (48 mM). After 20 min incubation at room temperature, 10 μl of bromo[1-14C]acetic acid (175 nmol; 10 μCi) were added, and the mixture was incubated 1 h at room temperature under argon. To quench the reaction, 20 μl of 0.1 M DTT followed by 60 μl of 1 M potassium phosphate (pH 8.0) were added. Guanidine⋅HCl (320 mg) was added after 5 min, and the enzyme was dialyzed 2.5 h against 1.5 liters of 20 mM Tris⋅HCl buffer (pH 8.0) and 1 mM EDTA under argon.
Tryptic Digestion of Carboxymethylated TR and Separation of Peptides.
The dialyzed protein was incubated with 20 μl of trypsin–N-1-tosylamido-2-phenylethyl chloromethyl ketone solution (2.8 mg/ml) for 4 h under argon and then was adjusted to pH 2.0 with HCOOH and loaded on a C18 HPLC column. Peptides were eluted with a 0–50% linear gradient of acetonitrile in 0.05% trifluoroacetic acid (0.5 ml/min for 100 min), and half-minute fractions were collected. The 75Se radioactivity of each fraction was detected by gamma counting, and a 10–20% aliquot of each fraction was analyzed for total radioactivity by liquid scintillation counting. The amount of 14C radioactivity was calculated by difference. Alkylation experiments performed at pH 8 used a similar procedure except the initial enzyme solution was adjusted to pH 8 with potassium phosphate and incubation time with bromoacetate was 30 min.
Amino Acid Analysis of the 14C-Alkylated Enzyme.
Fully active TR (1.14 nmol; 1.5 × 105 cpm in 100 μl) was reduced with NADPH and was alkylated with 5 μCi of bromo[1-14C]acetate at pH 6.5 as described above. In a parallel experiment, NADP+ was added instead of NADPH. After alkylation, the enzyme samples were dialyzed two times against 1 liter of 10% acetic acid and were hydrolyzed with 6 M HCl for 1h at 156°C under argon. Authentic carboxymethylselenocysteine (CMSecys) prepared from selenocystine (18) and carboxymethylcysteine (CMCys) (20 nmol of each) were added to each protein sample before hydrolysis. Amino acids in the hydrolysate were separated after PTIC derivatization (19) by HPLC analysis. The peak fractions corresponding to the Secys and Cys carboxymethylated derivatives were collected, and the radioactivity was measured as described above.
RESULTS
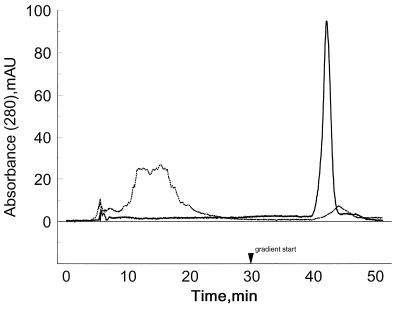
Thioredoxin reductase preparations, freshly isolated from cultured human lung adenocarcinoma cells and from cultured HeLa cells, contain enzyme forms that exhibit varying affinity to heparin (2, 4). To determine whether variations in oxygen availability to the cells during growth in stationary liquid cultures or in spinner flasks were responsible for the differences, the effect of oxygen level on TR synthesis was studied. By using a fermentor that allowed control of the dissolved oxygen content of the medium, HeLa cells were cultured either at a high oxygen level (50% dissolved oxygen saturation) that was slightly growth inhibitory or at a lower level (30% dissolved oxygen saturation) that proved to be optimum for growth. Enzyme preparations from cells grown at the optimal oxygen level did not bind to heparin affinity columns, and TR was recovered in the application buffer. The representative enzyme sample shown in Table 1, elution time 13 min, exhibited high activity on both disulfide substrates. In contrast, enzymes prepared from cells exposed to higher oxygen levels contained both high and low heparin affinity isoforms (Table 1). The enzyme form that bound to heparin and was eluted at 43 min by 400–500 mM KCl exhibited low catalytic activity and a decreased 75Se content. Similar results were obtained with enzyme preparations isolated from other batch cultures grown at the higher oxygen level, and in each instance, the low-activity form that was retained by heparin had ≈50% lower 75Se content. Although these results suggested that affinity to heparin was related to low activity and low selenium content, previous studies (2, 4) indicated that high heparin affinity TR isolated from human lung adenocarcinoma cells did not differ significantly in activity from the low heparin affinity isoforms. Evidence that a change in protein conformation independent of selenium content could be a determinant of TR affinity to heparin is shown in Fig. 1. In this experiment, fully active TR that did not bind to heparin was converted quantitatively to a high heparin affinity form by reduction with tris(2-carboxyethyl)phosphine. This fully reduced enzyme species retained 1 Se per subunit and exhibited full catalytic activity if strictly anaerobic conditions were maintained during recovery from the column (Table 2). However, this TCEP-reduced enzyme species, in contrast to an identical enzyme sample reduced with NADPH, lost both selenium and catalytic activity on exposure to oxygen-containing solutions. This marked sensitivity of TCEP-reduced enzyme to oxygen inactivation could be prevented by NADPH supplementation, suggesting that an enzyme conformation elicited by bound pyridine nucleotide confers stability to oxygen. Presumably any NADP+ originally bound to the enzyme was lost during chromatography of the TCEP-reduced sample on the HPLC heparin column in the presence of 1 mM TCEP. The possibility that an enzyme conformation resulting in exposure of basic groups was induced by conversion of TR to the fully reduced state can be considered to explain the observed change from low to high affinity for the sulfated heparin matrix. A conformational change induced by oxidative elimination of selenium from TR during growth of HeLa cells at elevated oxygen levels also could explain the high heparin affinity of the purified selenium-deficient, low-activity enzyme forms isolated from the population. In fact, electronic absorption spectra of the isolated low-activity, selenium-deficient enzyme indicated that the FAD cofactor bound to the enzyme was maintained in a partially reduced state that did not revert to the oxidized form characteristic of the fully active enzyme (data not shown).
Table 1.
Thioredoxin reductase from HeLa cells
| Retention time, min | Total protein, mg | 75Se, cpm/mg | Sp. activity, μmol/mg/min
|
|
|---|---|---|---|---|
| DTNB | Trx + glutathione disulfide* | |||
| Cells grown under 50% of saturation dissolved O2 | ||||
| 13 | 1.03 | 3.18 × 106 | 50.8 | 14.6 |
| 43 | 0.27 | 1.78 × 106 | 14.7 | 6.4 |
| Cells grown under 30% of saturation dissolved O2 | ||||
| 13 | 0.91 | 1.0 × 106 | 45.5 | 13.6 |
| 43 | 0 | |||
Isoforms are separated by heparin affinity chromatography.
Similar activity is observed with oxidized insulin as disulfide substrate.
Figure 1.
TCEP reduction of a low heparin affinity form of TR converts it to a high heparin affinity form. Low heparin affinity TR (≈70 μg) was rechromatographed on a heparin HPLC column in buffer A (dotted line). Another 70-μg sample was reduced with 1 mM TCEP in buffer A and was chromatographed on the same column equilibrated with 1 mM TCEP in buffer A (solid line). In both cases, after a 30 min wash with the application buffer, a linear KCl gradient (0–1 M) in the same buffer was applied for 20 min at a flow rate of 0.5 ml/min.
Table 2.
Enzyme properties after reduction
| Reductant | Experimental condition | Heparin affinity | 75Se, cpm/mg protein | Specific activity, μmol/min/mg
|
|
|---|---|---|---|---|---|
| DTNB | Trx + insulin | ||||
| None | Aerobic | Low | 1.1 × 106 | 44.8 | 13.6 |
| NADPH | Aerobic | High | 1.1 × 106 | 51.0 | 15.0 |
| TCEP | Aerobic | High | 0.6 × 106 | 14.6 | 6.6 |
| TCEP | Anaerobic | High | 1.0 × 106 | 42.5 | 12.0 |
| TCEP* | Aerobic | High | 0.9 × 106 | 42.2 | — |
NADPH, 5 mM or TCEP, 1 mM was used.
After elution from the heparin column, the sample was supplemented with 1 mM NADPH before buffer transfer and concentration.
TR Activity Profile as a Function of pH with DTNB as Substrate.
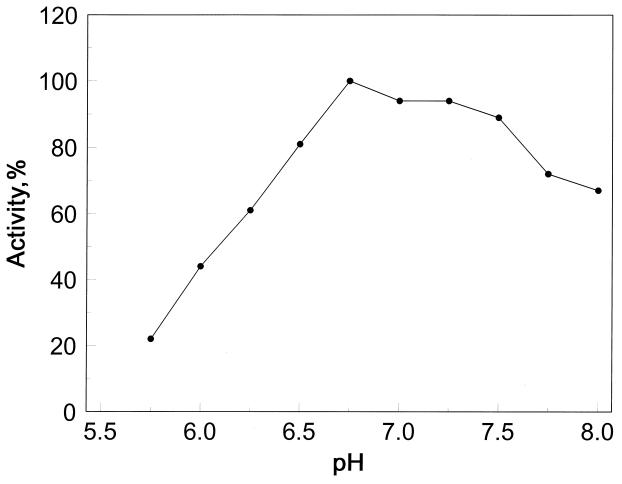
The pH activity profile of TR with DTNB as disulfide substrate in Fig. 2 shows that TR is active over a broad pH range of 6.5 to 8, with a maximum between 6.8 and 7.2. In this pH range, the selenocysteine residue (nominal pKa of 5.3) is fully ionized and therefore fully active.
Figure 2.
DTNB reduction by TR as a function of pH. Potassium phosphate buffers (100 mM) of the indicated pH were used.
Alkylation of C-Terminal Redox Center.
The effect of alkylation on the activity of human TR and the identities of the residues derivatized were determined as a means of establishing a role of the selenocysteine residue located in the putative redox center consisting of Cys-497 and Secys-498 at the C-terminus of each protein subunit. The reaction was carried out at pH 6.5, and bromoacetate was used instead of iodoacetate to make alkylation more selective for the fully ionized selenol group of selenocysteine. However, if the pKa of the thiol group of the adjacent cysteine residue is abnormally low, it also might be alkylated under these conditions. Fully active 75Se-labeled TR, after reduction with NADPH and reaction with bromo[1-14C]acetate, was converted to a 99% inactive enzyme form that contained 1.1 equivalents of alkyl group per subunit. Amino acid analysis of the alkylated enzyme, after acid hydrolysis, showed that doubly labeled CM-Secys accounted for at least 80% of the recovered alkyl group and that 20% or less was in CM-Cys (Table 3). The amount of CM-Secys, when corrected for losses caused by the marked oxygen lability of the selenoether, corresponded to complete derivatization of 1 Secys-498 per subunit. In subsequent experiments, we found that the amount of alkyl group recovered in CM-Cys, which proved to be alkylated Cys-497, was closer to 5% under these conditions than the 20% shown in Table 3. Unless carefully protected from oxidative decomposition, there is greater loss of the more labile selenoether than the thioether derivative, with the result that the amount of cysteine derivatized is overestimated. As a control, enzyme treated with NADP+ instead of NADPH was carried through the same procedure. The amount of alkyl group incorporated (0.25 equivalent per subunit; Table 3) recovered in both CM-Secys and CM-Cys indicates that the isolated enzyme preparation contained partially reduced enzyme species.
Table 3.
Alkylation of 75Se-labeled TR with bromo[1−14C]acetate. HPLC analysis of acid hydrolysates
| Enzyme treatment | Total 75Se, cpm | Total 14C, cpm |
|---|---|---|
| NADPH | ||
| Applied to column | 32,060 | 32,730 |
| CM-cysteine | (270) | 3,940 (19%) |
| CM-Se-cysteine | 19,170 | 16,330 (81%) |
| NADP+ | ||
| Applied to column | 48,520 | 12,720 |
| CM-cysteine | (300) | 2,940 (42%) |
| CM-Se-cysteine | 4,620* | 4,100 (58%) |
1.1 equivalent alkyl group incorporated/mol subunit of NADPH-reduced enzyme; 0.25 equivalent alkyl group incorporated/mol subunit NADP+ enzyme control.
14% of the applied 75Se emerged early from the column, and an additional 36% was recovered later in the profile after the CM-Se-cysteine peak.
Location of Alkylated Amino Acids in Peptides by Edman Degradation.
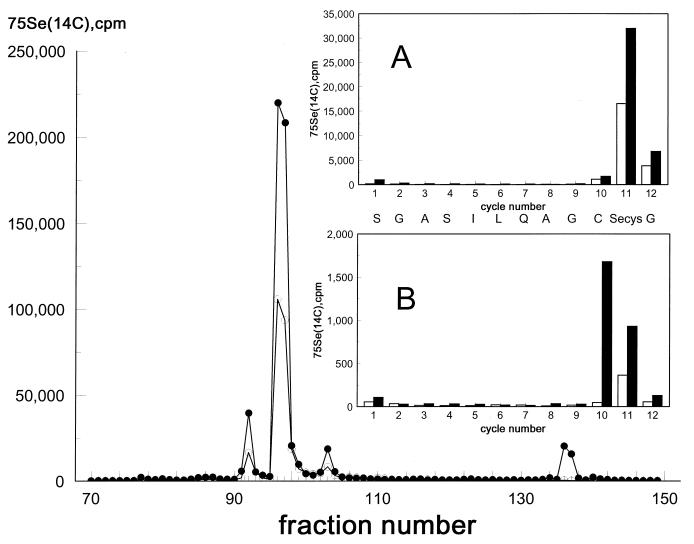
The location of the cysteine residue that was alkylated at pH 6.5 after NADPH reduction of native TR was determined by Edman degradation of 14C- and 75Se-labeled C-terminal tryptic peptides. As shown in the C18 HPLC profile of Fig. 3, one major and two minor peptide fractions contained both isotopes and accounted for >75% of the radioactivity applied to the column. Sequence analysis of the major peptide (Fig. 3A) showed that the predicted amino acid emerged at each cycle of the 12-residue peptide (3, 4) and that the presence of both 75Se and 14C in cycle 11 corresponded to CM-Secys. The absence of 14C in cycle 10 corresponding to the position of the adjacent Cys-497 indicates that no CM-Cys was present in the major peptide. Analysis of the minor preceding peptide showed that 14C emerged in cycle 10 and both 14C and 75Se emerged in cycle 11, indicating that the two adjacent amino acid residues Cys-497 and Secys-498 in the protein subunit had been alkylated. Because of exposure of this peptide to air before sequence analysis, there was considerable loss of the CM-Secys derivative as evidenced by the appearance of decomposition products containing both isotopes in cycle l (data not shown). Another doubly labeled peptide of the same size that eluted at the same HPLC position was obtained from enzyme alkylated at pH 8. The sequence of this peptide is shown in Fig. 3B. In this case, the absence of radioactivity in cycle 1 indicates that the CM-Secys had not undergone oxidative decomposition before analysis. The appearance of 14C in cycle 10 and both 14C and 75Se in cycle 11 clearly shows that the alkyl derivatives of the adjacent Cys-497 and Secys-498 residues were present in the peptide. However, the total CM-Cys-497 was still low, accounting for only ≈7% of the total alkyl group incorporated in the protein at pH 8. The similar extent of alkylation of Cys-497 at pH 6.5 and at pH 8 suggests that interference caused by steric constraints imposed by rapid reaction of Secys-498 with bromoacetate might be a factor, as has been suggested for incomplete alkylation of adjacent cysteine residues in mercuric reductase (20). It is of interest that the redox active disulfide center of TR comprising Cys-59 and Cys-64 also was alkylated partially by bromoacetate at pH 8. The sequence of the minor 14C-labeled tryptic peptide that was eluted in fractions 139–140 of the HPLC profile was determined to be W G L G G T C V N V G X. This sequence is identical to the deduced sequence of residues 53 to 64 from human placental TR (5). CM-Cys that eluted in cycle 7 corresponded to Cys-59 and contained ≈70% of the 14C incorporated in the peptide. The remaining 30% was found in cycle 12 at the position of Cys-64 (X in the sequence above). This minor peptide comprising the cysteine disulfide center of the enzyme accounted for ≈20% of the [14C]alkyl group incorporated in the protein at pH 8. The incorporation of alkyl group in this peptide at pH 6.5 was lower, estimated to be 6% of the total amount incorporated. The results of these alkylation experiments, carried out with NADPH reduced native enzyme, show that in this conformation the cysteine residues of the active site disulfide in the amino terminal region of the protein are only weakly reactive, either at pH 6.5 or at pH 8, whereas the Secys-498 residue in the C-terminal redox center reacts completely under both conditions. The fact that the low extent of alkylation of Cys-497 observed at pH 6.5 was not increased at pH 8 implies that the initial introduction of a carboxymethyl group on the very reactive Secys-498 residue may have introduced a steric constraint on reaction of the adjacent ionized thiol group of Cys-497. If this is the explanation of the apparent low reactivity of Cys-497 with bromoacetate, its conversion to CM-Cys at pH 6.5, even though partial, indicates that it has a pKa value lower than the nominal 8.3 for cysteine and thus should be ionized and redox-active throughout the pH activity range of the enzyme. The conditions imposed by the introduction of an artificial carboxymethyl group on the Secys of the C-terminal redox center are very different from those of the normal electron transfer process. Therefore, under enzyme turnover conditions, Cys-497 could be fully reactive rather than only partially reactive, as was the case in the alkylation experiments.
Figure 3.
Reversed-phase HPLC separation of a tryptic digest of bromo[1-14C]acetate alkylated 75Se-labeled TR from HeLa cells. Conditions for alkylation, digestion with trypsin, and HPLC separation are described in Materials and Methods. Solid circles, 14C radioactivity; open circles, 75Se radioactivity. (A) Edman degradation profile of 14C [solid bars] and 75Se [open bars] recovered at each sequencer cycle from the major tryptic peptide eluted in fractions 96–97. (B) Profile as above from a minor peptide eluted in fractions 91–92. The amino acid identified in each cycle of A and B, shown in the line between the inserts, is the same as the predicted sequence of human placenta TR (5).
DISCUSSION
Mammalian TR is distinguished from a family of NADPH-dependent, FAD-containing disulfide reductases that include glutathione reductase and lipoamide reductase by the presence of a C-terminal peptide extension containing a selenocysteine residue in the penultimate position (3, 4, 5, 21). The presence of a selenol group that is fully ionized at physiological pH and is highly reactive undoubtedly explains the unusual, wide substrate specificity of the mammalian enzyme as contrasted to TRs from E. coli, yeast, and some other lower eukaryotes that are not selenoproteins. It is interesting that the level of TR in two transformed human cell lines and some other mammalian cells (2, 4, 22) is considerably higher than that reported for normal tissues from a variety of sources (4, 14, 15, 21). Thus, the lower redox potential characteristic of rapidly growing tumor cells can be a function of higher TR activity in addition to the high reduced glutathione levels present. Based on the well known fact that the low pKa of a free selenol ensures that it will be fully ionized at physiological pH, it is not surprising that the penultimate selenocysteine residue at the C-terminus of thioredoxin reductase is highly reactive with alkylating agents. The fact that enzyme activity is inhibited completely by the introduction of 1.1 alkyl groups per subunit, most of which are located on the selenocysteine residue of the subunit, provides strong evidence of the essential role of a selenol in the catalytic activity of human thioredoxin reductase. Furthermore, it was shown that enzyme preparations isolated from HeLa cells cultured under higher-than-optimum oxygen levels invariably contained appreciable amounts of an enzyme form that was 50% lower in selenium content and 50% as active catalytically as the normal enzyme. The consistent correlation of a lower selenium content with lower catalytic activity of enzyme forms present in the various preparations also shows that the intact selenocysteine residue is required for enzyme activity. Some evidence that the Cys-497 residue in the C-terminal redox center also has a direct role in catalysis is provided by the alkylation experiments. A minor peptide isolated from native reduced enzyme, alkylated at either pH 6.5 or at pH 8.0, contained both CMCys-497 and CMSecys-498, showing that the thiol group as well as the selenol was ionized under these conditions. The lack of pH dependency on CMCys-497 formation indicates an abnormal pKa value for this cysteine residue and suggests that steric constraints imposed by rapid introduction of an alkyl group on the adjacent Secys could account for the apparent low reactivity of Cys-497.
We reported (2–4) that mammalian TRs isolated from cultured human lung adenocarcinoma cells, HeLa cells, and human T-cells actually were populations of enzyme species that varied in reactivity with heparin affinity matrices and with polyclonal antibodies elicited to rat liver TR in immunoblot assays. The present study clearly shows that a fully active enzyme preparation that failed to bind to heparin was converted on reduction with either NADPH or TCEP to a conformational state that exhibited high affinity to heparin. A possible explanation is that basic groups become exposed in the reduced enzyme species that render the protein reactive with sulfate groups of the heparin matrix. Both the original oxidized form of the enzyme and the fully reduced species contained a full complement of Se. In contrast to the NADPH-reduced enzyme that was stable to subsequent exposure to oxygen and retained full activity, the TCEP-reduced enzyme was unstable under the same conditions and showed parallel loss of Se and activity unless supplemented with NADPH. In the absence of added pyridine nucleotide, anaerobic conditions with rigorous exclusion of dissolved oxygen were required to maintain stability of the TCEP reduced enzyme. A pyridine nucleotide-induced conformational form that protects the selenocysteine residue from oxidation and Se elimination under aerobic conditions is indicated, which is consistent with statements in the literature (23) that the stability of mammalian TR is favored by the presence of bound NADP+. In the present study, TR preparations isolated from HeLa cells grown at a higher than optimum oxygen concentration repeatedly were found to contain varying amounts of selenium deficient species that exhibited low catalytic activity. This enzyme form that was separated on the basis of affinity to heparin usually had a 50% lower selenium content and exhibited half the normal enzyme activity, suggesting that oxidative decomposition of selenocysteine in the protein had occurred during growth under high oxygen conditions. The reason for the failure to find enzyme with <50% of normal Se content and even lower activity is not apparent unless there is a conformation state that protects a selenocysteine residue on one subunit of the homodimeric enzyme after elimination of selenium from the corresponding residue in the other subunit. Alternatively, premature termination at the UGA codon of the message during enzyme synthesis might occur if selenocysteyl-tRNA is limiting at the higher oxygen levels. In this case, some subunits lacking selenocysteine and terminating in cysteine would result. Formation of the dimeric 50% active enzyme species then would involve association of one shortened and one full-length subunit. These possibilities could be resolved by C-terminal sequence analyses of the separated subunits. A C-terminal cysteine would indicate termination at UGA whereas, with selenium elimination, the C-terminal sequence is Cys-Aminoacrylate (or Ser)-Gly.
ABBREVIATIONS
- TR
thioredoxin reductase
- Cys
cysteine
- Secys
selenocysteine
- CM
carboxymethyl
- TCEP
Tris-(2-carboxyethyl)phosphine
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
References
- 1.Holmgren A. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 2.Tamura T, Stadtman T C. Proc Natl Acad Sci USA. 1996;93:1006–1011. doi: 10.1073/pnas.93.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gladyshev V N, Jeang K-T, Stadtman T C. Proc Natl Acad Sci USA. 1996;93:6146–6151. doi: 10.1073/pnas.93.12.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S-Y, Stadtman T C. Proc Natl Acad Sci USA. 1997;94:6138–6141. doi: 10.1073/pnas.94.12.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasdaska P Y, Gasdaska J R, Cochran S, Powis G. FEBS Lett. 1995;373:5–9. doi: 10.1016/0014-5793(95)01003-w. [DOI] [PubMed] [Google Scholar]
- 6.Thelander L, Reichard P. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- 7.Spyrou G, Bjornstedt M, Kumar S, Holmgren A. FEBS Lett. 1995;368:59–63. doi: 10.1016/0014-5793(95)00599-5. [DOI] [PubMed] [Google Scholar]
- 8.Handel M L, Watts C L, DeFazio A, Day R O, Sutherland R L. Proc Natl Acad Sci USA. 1995;92:4497–4501. doi: 10.1073/pnas.92.10.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makropoulos V, Bruning T, Schulze-Osthoff K. Arch Toxicol. 1996;70:277–283. doi: 10.1007/s002040050274. [DOI] [PubMed] [Google Scholar]
- 10.Kim I Y, Stadtman T C. Proc Natl Acad Sci USA. 1997;94:12904–12907. doi: 10.1073/pnas.94.24.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman R B, Nandi D L. Biochem Biophys Res Commun. 1988;155:1248–1254. doi: 10.1016/s0006-291x(88)81274-9. [DOI] [PubMed] [Google Scholar]
- 12.Chae H Z, Chung S J, Rhee S G. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 13.Bjornstedt M, Xue J, Huang W, Akesson B, Holmgren A. J Biol Chem. 1994;269:29382–29384. [PubMed] [Google Scholar]
- 14.Oblong J E, Gasdaska P Y, Sherrill K, Powis G. Biochemistry. 1993;32:7271–7277. doi: 10.1021/bi00079a025. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Galisteo E, Padilla C A, Garcia-Alfonso C, Lopez-Barea J, Barcena J A. Biochimie (Paris) 1993;75:803–809. doi: 10.1016/0300-9084(93)90131-b. [DOI] [PubMed] [Google Scholar]
- 16.Marcocci L, Flohe L, Packer L. Biofactors. 1997;6:351–358. doi: 10.1002/biof.5520060305. [DOI] [PubMed] [Google Scholar]
- 17.Gallegos A, Berggren M, Gasdaska J R, Powis G. Cancer Res. 1997;57:4965–4970. [PubMed] [Google Scholar]
- 18.Stadtman T C. Methods Enzymol. 1984;107:576–581. doi: 10.1016/0076-6879(84)07041-5. [DOI] [PubMed] [Google Scholar]
- 19.Bidlingmeyer B A, Cohen S A, Tarvin T L. J Chromatogr. 1984;336:93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- 20.Miller S M, Moore M J, Massey V, Williams C H, Jr, Distefano M D, Ballou D P, Walsh C T. Biochemistry. 1989;89:1194–1205. doi: 10.1021/bi00429a037. [DOI] [PubMed] [Google Scholar]
- 21.Zhong L, Arner E S J, Ljung J, Aslund F, Holmgren A. J Biol Chem. 1998;273:8581–8591. doi: 10.1074/jbc.273.15.8581. [DOI] [PubMed] [Google Scholar]
- 22.Tamura T, Gladyshev V, Liu S-Y, Stadtman T C. Biofactors. 1996;5:99–102. [PubMed] [Google Scholar]
- 23.Arscott L D, Gromer S, Schirmer R H, Becker K, Williams C H., Jr Proc Natl Acad Sci USA. 1997;94:3621–3626. doi: 10.1073/pnas.94.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]