Abstract
Recently a dapF mutant of Escherichia coli lacking the diaminopimelate epimerase was found to have an unusual large LL-diaminopimelic acid (LL-DAP) pool as compared with that of meso-DAP (C. Richaud, W. Higgins, D. Mengin-Lecreulx, and P. Stragier, J. Bacteriol. 169:1454-1459, 1987). In this report, the consequences of high cellular LL-DAP/meso-DAP ratios on the structure and metabolism of peptidoglycan were investigated. For this purpose new efficient high-pressure liquid chromatography techniques for the separation of the DAP isomers were developed. Sacculi from dapF mutants contained a high proportion of LL-DAP that varied greatly with growth conditions. The same was observed with the two DAP-containing precursors, UDP-N-acetylmuramyl-tripeptide and UDP-N-acetylmuramyl-pentapeptide. The limiting steps for the incorporation of LL-DAP into peptidoglycan were found to be its addition to UDP-N-acetylmuramyl-L-alanyl-D-glutamate and the formation of the D-alanyl-DAP cross-bridges. The Km value of the DAP-adding enzyme for LL-DAP was 3.6 x 10(-2) M as compared with 1.1 x 10(-5) M for meso-DAP. When isolated sacculi were treated with Chalaropsis N-acetylmuramidase and the resulting soluble products were analyzed by high-pressure liquid chromatography, the proportion of the main peptidoglycan dimer was lower in the dapF mutant than in the parental strain. Moreover, the proportion of LL-DAP was higher in the main monomer than in the main dimer, where it was almost exclusively located in the donor unit. There are thus very few D-alanyl-LL-DAP cross-bridges, if any. We also observed that large amounts of LL-DAP and N-succinyl-LL-DAP were excreted in the growth medium by the dapF mutant.
Full text
PDF
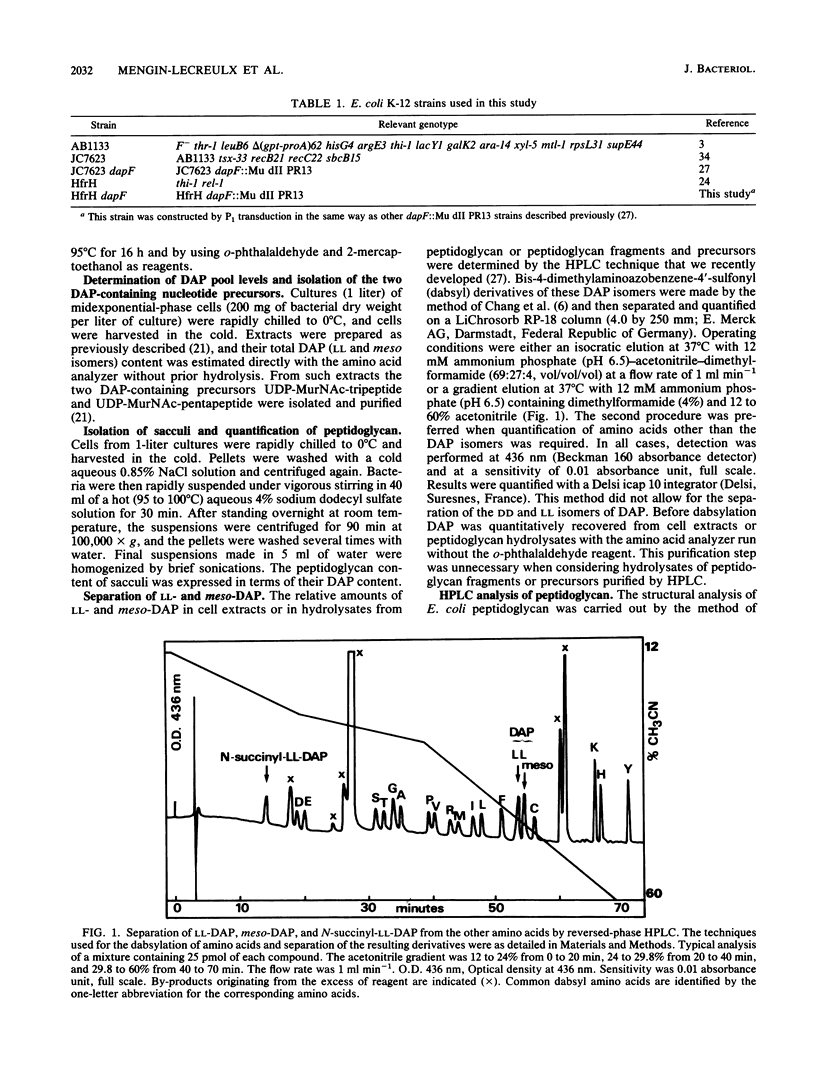


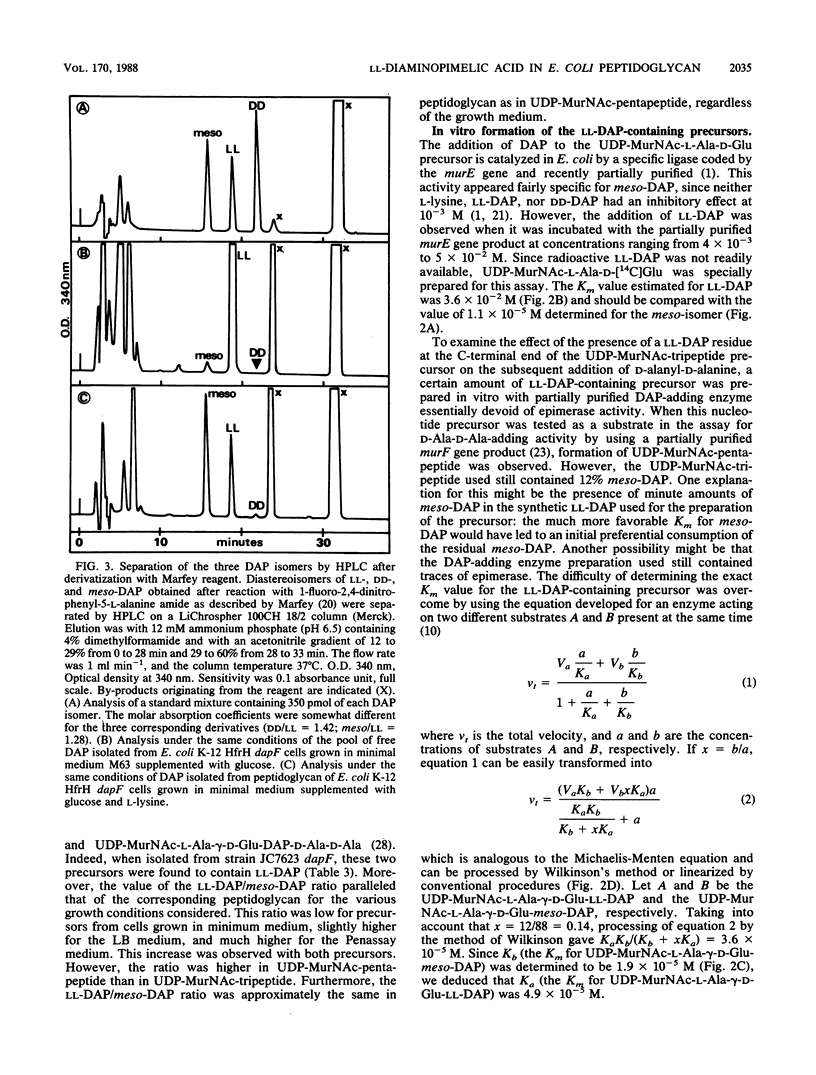




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTIA M., HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid. III. Properties and distribution of diaminopimelic acid racemase, an enzyme causing interconversion of the LL and meso isomers. Biochem J. 1957 Mar;65(3):448–459. doi: 10.1042/bj0650448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-Ghalia M., Michaud C., Blanot D., van Heijenoort J. Specificity of the uridine-diphosphate-N-acetylmuramyl-L-alanyl-D-glutamate: meso-2,6-diaminopimelate synthetase from Escherichia coli. Eur J Biochem. 1985 Nov 15;153(1):81–87. doi: 10.1111/j.1432-1033.1985.tb09269.x. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Park J. T. Molecular model for elongation of the murein sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1844–1848. doi: 10.1073/pnas.81.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Reichler J., Park J. T. Evidence for multisite growth of Escherichia coli murein involving concomitant endopeptidase and transpeptidase activities. J Bacteriol. 1983 Oct;156(1):386–392. doi: 10.1128/jb.156.1.386-392.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Y., Knecht R., Braun D. G. Amino acid analysis at the picomole level. Application to the C-terminal sequence analysis of polypeptides. Biochem J. 1981 Dec 1;199(3):547–555. doi: 10.1042/bj1990547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. Biosynthetic interrelations of lysine, diaminopimelic acid, and threonine in mutants of Escherichia coli. Nature. 1952 Mar 29;169(4300):534–536. doi: 10.1038/169534a0. [DOI] [PubMed] [Google Scholar]
- Day A., White P. J. Enzymic assays for isomers of 2,6-diaminopimelic acid in walls of Bacillus cereus and Bacillus megaterium. Biochem J. 1977 Mar 1;161(3):677–685. doi: 10.1042/bj1610677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouret B., Mengin-Lecreulx D., van Heijenoort J. Reverse-phase high-pressure liquid chromatography of uridine diphosphate N-acetylmuramyl peptide precursors of bacterial cell wall peptidoglycan. Anal Biochem. 1981 Jun;114(1):59–63. doi: 10.1016/0003-2697(81)90451-6. [DOI] [PubMed] [Google Scholar]
- GILVARG C. N-Succinyl-L-diaminopimelic acid. J Biol Chem. 1959 Nov;234:2955–2959. [PubMed] [Google Scholar]
- GILVARG C. N-succinyl-L-diamino-pimelic acid, an intermediate in the biosynthesis of diaminopimelic acid. Biochim Biophys Acta. 1957 Apr;24(1):216–217. doi: 10.1016/0006-3002(57)90176-2. [DOI] [PubMed] [Google Scholar]
- Girodeau J. M., Agouridas C., Masson M., Pineau R., Le Goffic F. The lysine pathway as a target for a new genera of synthetic antibacterial antibiotics? J Med Chem. 1986 Jun;29(6):1023–1030. doi: 10.1021/jm00156a021. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Essig P., Martin H. H. Characterization of minor fragments after digestion of Escherichia coli murein with endo-N,O-diacetylmuramidase from Chalaropsis, and determination of glycan chain length. FEBS Lett. 1982 Feb 8;138(1):109–112. doi: 10.1016/0014-5793(82)80406-7. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Cleavage and resynthesis of peptide cross bridges in Escherichia coli murein. J Bacteriol. 1983 Oct;156(1):136–140. doi: 10.1128/jb.156.1.136-140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid: their distribution in nature and behaviour towards certain enzyme preparations. Biochem J. 1955 Dec;61(4):562–568. doi: 10.1042/bj0610562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyh-Bouille M., Bonaly R., Ghuysen J. M., Tinelli R., Tipper D. LL-diaminopimelic acid containing peptidoglycans in walls of Streptomyces sp. and of Clostridium perfringens (type A). Biochemistry. 1970 Jul 21;9(15):2944–2952. doi: 10.1021/bi00817a002. [DOI] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1109–1117. doi: 10.1128/jb.151.3.1109-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., van Heijenoort J. Effect of growth conditions on peptidoglycan content and cytoplasmic steps of its biosynthesis in Escherichia coli. J Bacteriol. 1985 Jul;163(1):208–212. doi: 10.1128/jb.163.1.208-212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud C., Blanot D., Flouret B., Van Heijenoort J. Partial purification and specificity studies of the D-glutamate-adding and D-alanyl-D-alanine-adding enzymes from Escherichia coli K12. Eur J Biochem. 1987 Aug 3;166(3):631–637. doi: 10.1111/j.1432-1033.1987.tb13560.x. [DOI] [PubMed] [Google Scholar]
- Pisabarro A. G., de Pedro M. A., Vázquez D. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J Bacteriol. 1985 Jan;161(1):238–242. doi: 10.1128/jb.161.1.238-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richaud C., Higgins W., Mengin-Lecreulx D., Stragier P. Molecular cloning, characterization, and chromosomal localization of dapF, the Escherichia coli gene for diaminopimelate epimerase. J Bacteriol. 1987 Apr;169(4):1454–1459. doi: 10.1128/jb.169.4.1454-1459.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heijenoort J., Elbaz L., Dezélée P., Petit J. F., Bricas E., Ghuysen J. M. Structure of the meso-diaminopimelic acid containing peptidoglycans in Escherichia coli B and Bacillus megaterium KM. Biochemistry. 1969 Jan;8(1):207–213. doi: 10.1021/bi00829a030. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman J. S., Nichols J. S. Purification and properties of diaminopimelic acid epimerase from Escherichia coli. J Biol Chem. 1984 Jul 25;259(14):8907–8914. [PubMed] [Google Scholar]
- de Pedro M. A., Schwarz U. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5856–5860. doi: 10.1073/pnas.78.9.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]


