Abstract
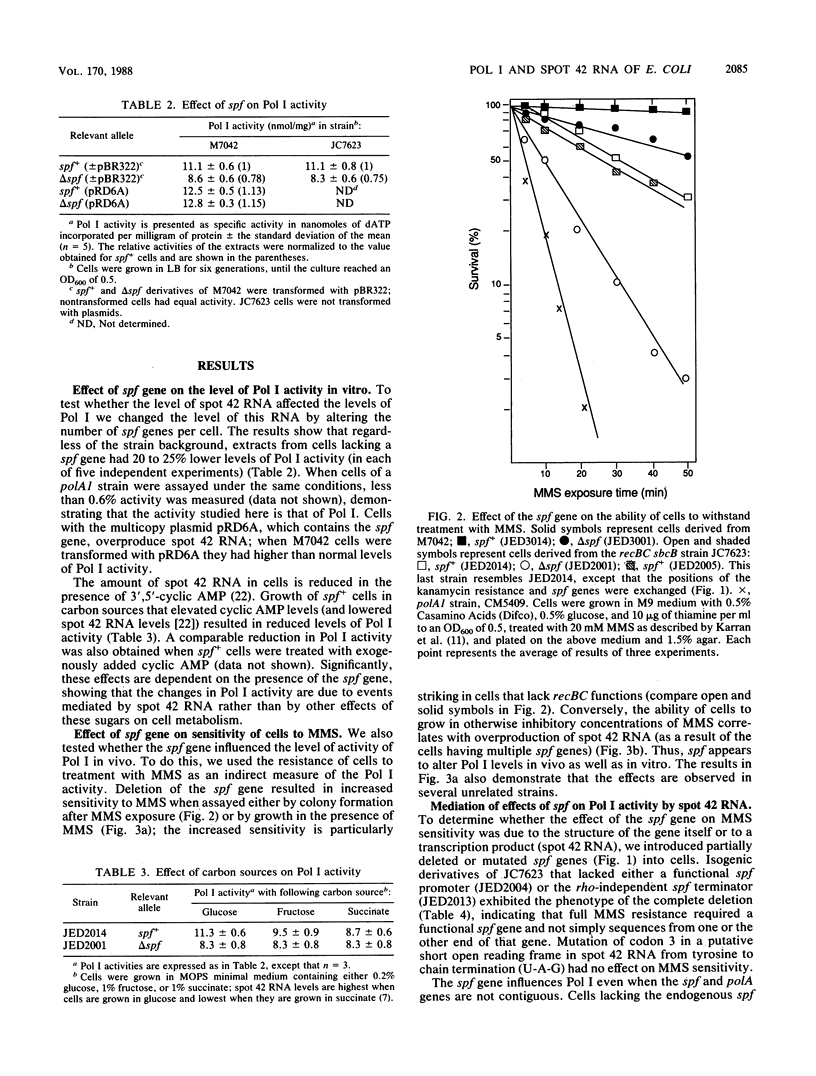
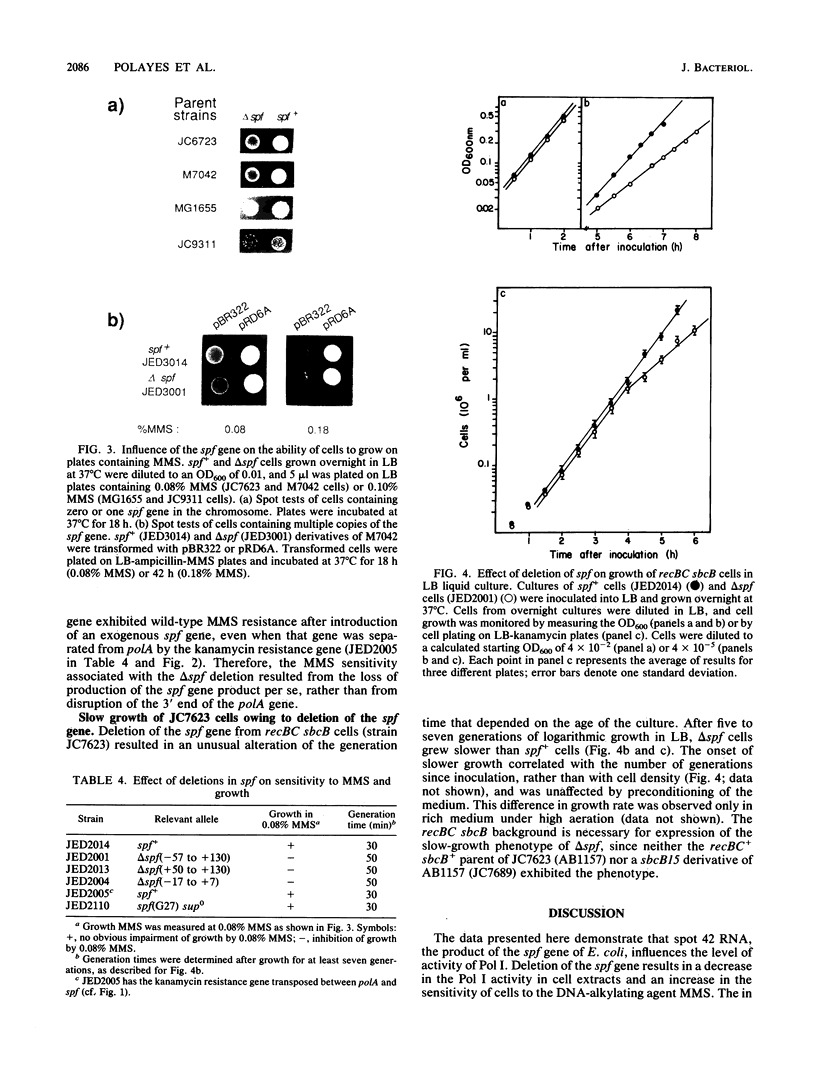
We have shown that the level of DNA polymerase I (Pol I) activity in Escherichia coli is influenced by the level of a 109-nucleotide RNA, spot 42 RNA. Deletion of the gene for spot 42 RNA results in a 20 to 25% decrease in Pol I activity, as assayed by nucleotide incorporation in cell extracts and a decrease in the ability of cells to grow in the presence of the DNA-alkylating agent methyl methanesulfonate. Also, a physiological reduction of the level of spot 42 RNA, by growth in media containing poor carbon sources, results in a corresponding decrease in Pol I activity. Conversely, overproduction of spot 42 RNA results in a 10 to 15% increase in Pol I activity in vitro. Thus, changes in the amount of spot 42 RNA result in relatively small but significant changes in Pol I activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Hatfull G. F., Joyce C. M. Deletion of the spf (spot 42 RNA) gene of Escherichia coli. J Bacteriol. 1986 Jun;166(3):746–750. doi: 10.1128/jb.166.3.746-750.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Schimmel P. Deletion of an essential gene in Escherichia coli by site-specific recombination with linear DNA fragments. J Bacteriol. 1984 Aug;159(2):783–786. doi: 10.1128/jb.159.2.783-786.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce C. M., Grindley N. D. Identification of two genes immediately downstream from the polA gene of Escherichia coli. J Bacteriol. 1982 Dec;152(3):1211–1219. doi: 10.1128/jb.152.3.1211-1219.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce C. M., Grindley N. D. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984 May;158(2):636–643. doi: 10.1128/jb.158.2.636-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran P., Lindahl T., Ofsteng I., Evensen G. B., Seeberg E. Escherichia coli mutants deficient in 3-methyladenine-DNA glycosylase. J Mol Biol. 1980 Jun 15;140(1):101–127. doi: 10.1016/0022-2836(80)90358-7. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Kushner S. R. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. doi: 10.1128/jb.120.3.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rice P. W., Dahlberg J. E. A gene between polA and glnA retards growth of Escherichia coli when present in multiple copies: physiological effects of the gene for spot 42 RNA. J Bacteriol. 1982 Dec;152(3):1196–1210. doi: 10.1128/jb.152.3.1196-1210.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. W., Polayes D. A., Dahlberg J. E. Spot 42 RNA of Escherichia coli is not an mRNA. J Bacteriol. 1987 Aug;169(8):3850–3852. doi: 10.1128/jb.169.8.3850-3852.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagan B. G., Dahlberg J. E. A small, unstable RNA molecule of Escherichia coli: spot 42 RNA. I. Nucleotide sequence analysis. J Mol Biol. 1979 Jul 5;131(3):573–592. doi: 10.1016/0022-2836(79)90008-1. [DOI] [PubMed] [Google Scholar]
- Sahagan B. G., Dahlberg J. E. A small, unstable RNA molecule of Escherichia coli: spot 42 RNA. II. Accumulation and distribution. J Mol Biol. 1979 Jul 5;131(3):593–605. doi: 10.1016/0022-2836(79)90009-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. DNA polymerase I from Escherichia coli. Methods Enzymol. 1974;29:3–12. doi: 10.1016/0076-6879(74)29003-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Ward D. F., Murray N. E. Construction and characterization of Escherichia coli polA-lacZ gene fusions. J Bacteriol. 1980 Jun;142(3):962–972. doi: 10.1128/jb.142.3.962-972.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]



