Abstract
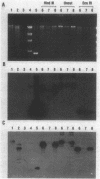
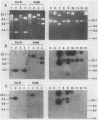
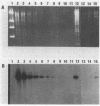
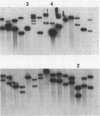
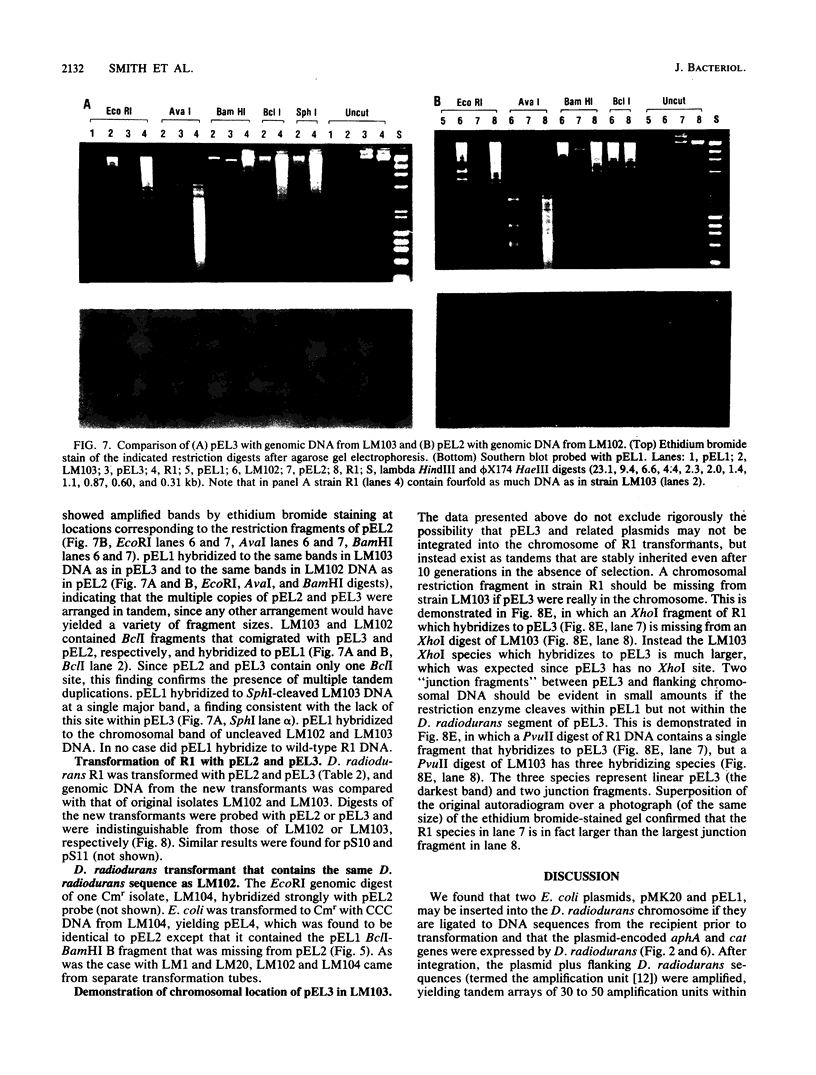
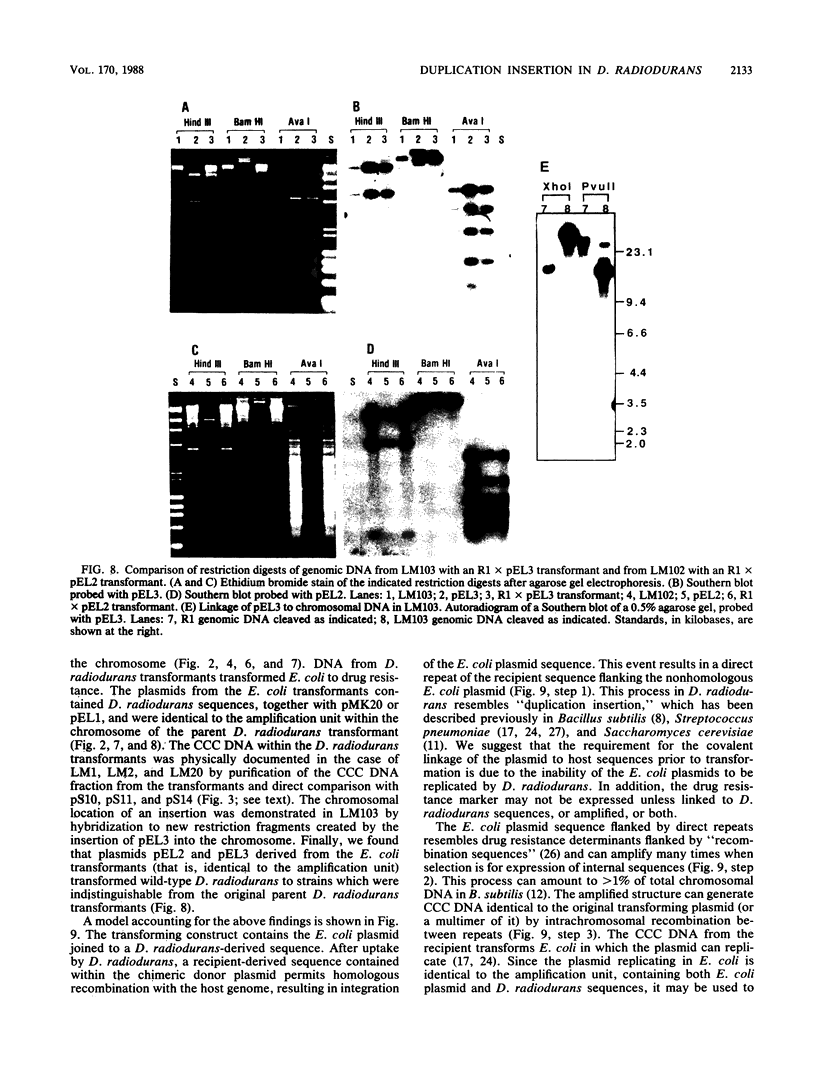
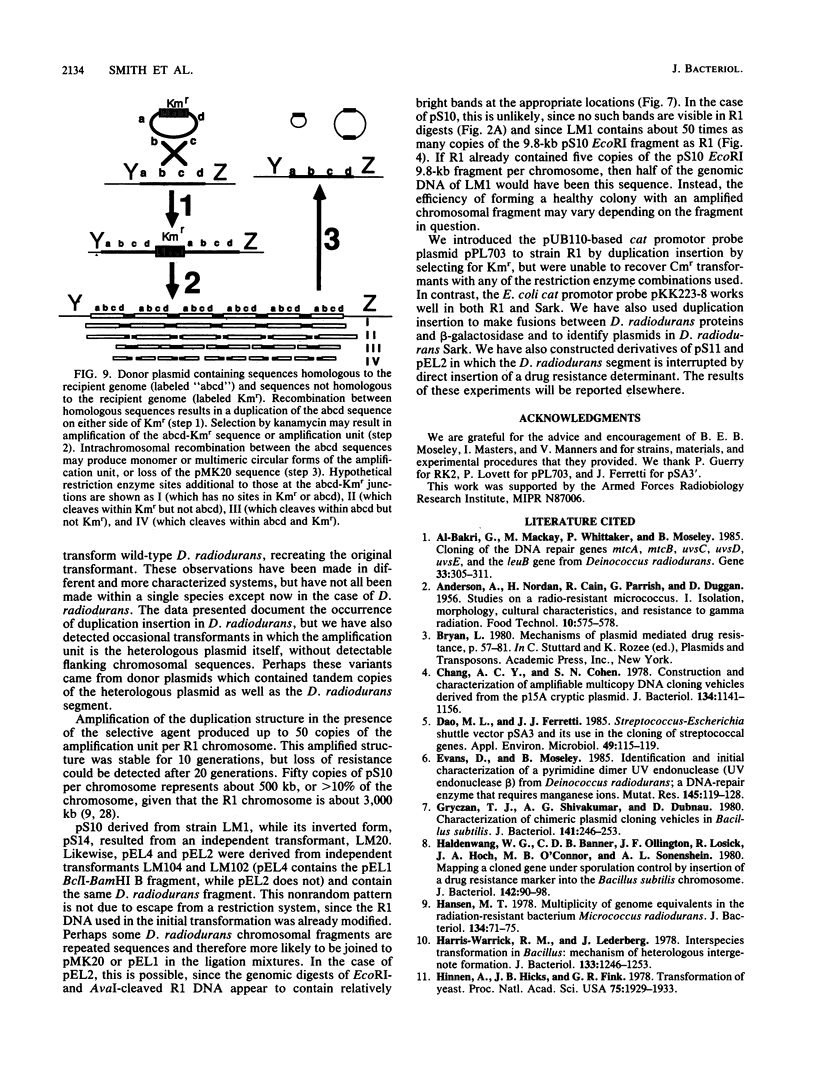
Escherichia coli drug resistance plasmids were introduced into Deinococcus radiodurans by cloning D. radiodurans DNA into the plasmids prior to transformation. The plasmids were integrated into the chromosome of the transformants and flanked by a direct repeat of the cloned D. radiodurans segment. The plasmid and one copy of the flanking chromosomal segment constituted a unit ("amplification unit") which was found repeated in tandem at the site of chromosomal integration. Up to 50 copies of the amplification unit were present per chromosome, accounting for approximately 10% of the genomic DNA. Circular forms of the amplification unit were also present in D. radiodurans transformants. These circles were introduced into E. coli, where they replicated as plasmids. The drug resistance determinants which have been introduced into D. radiodurans in this fashion are cat (from Tn9) and aphA (from Tn903). Transformation of D. radiodurans to drug resistance was efficient when the donor DNA was from D. radiodurans or E. coli, but was greatly reduced when the donor DNA was linearized with restriction enzymes prior to transformation. In the course of the study, a plasmid, pS16, was discovered in D. radiodurans R1, establishing that all Deinococcus strains so far examined contain plasmids.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bakri G. H., Mackay M. W., Whittaker P. A., Moseley B. E. Cloning of the DNA repair genes mtcA, mtcB, uvsC, uvsD, uvsE and the leuB gene from Deinococcus radiodurans. Gene. 1985;33(3):305–311. doi: 10.1016/0378-1119(85)90238-0. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao M. L., Ferretti J. J. Streptococcus-Escherichia coli shuttle vector pSA3 and its use in the cloning of streptococcal genes. Appl Environ Microbiol. 1985 Jan;49(1):115–119. doi: 10.1128/aem.49.1.115-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. M., Moseley B. E. Identification and initial characterisation of a pyrimidine dimer UV endonuclease (UV endonuclease beta) from Deinococcus radiodurans; a DNA-repair enzyme that requires manganese ions. Mutat Res. 1985 May;145(3):119–128. doi: 10.1016/0167-8817(85)90018-5. [DOI] [PubMed] [Google Scholar]
- Gryczan T., Shivakumar A. G., Dubnau D. Characterization of chimeric plasmid cloning vehicles in Bacillus subtilis. J Bacteriol. 1980 Jan;141(1):246–253. doi: 10.1128/jb.141.1.246-253.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Banner C. D., Ollington J. F., Losick R., Hoch J. A., O'Connor M. B., Sonenshein A. L. Mapping a cloned gene under sporulation control by inserttion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980 Apr;142(1):90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. T. Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol. 1978 Apr;134(1):71–75. doi: 10.1128/jb.134.1.71-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Lederberg J. Interspecies transformation in Bacillus: mechanism of heterologous intergenote transformation. J Bacteriol. 1978 Mar;133(3):1246–1253. doi: 10.1128/jb.133.3.1246-1253.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannière L., Niaudet B., Pierre E., Ehrlich S. D. Stable gene amplification in the chromosome of Bacillus subtilis. Gene. 1985;40(1):47–55. doi: 10.1016/0378-1119(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay M. W., al-Bakri G. H., Moseley B. E. The plasmids of Deinococcus spp. and the cloning and restriction mapping of the D. radiophilus plasmid pUE1. Arch Microbiol. 1985 Feb;141(1):91–94. doi: 10.1007/BF00446746. [DOI] [PubMed] [Google Scholar]
- Mongkolsuk S., Chiang Y. W., Reynolds R. B., Lovett P. S. Restriction fragments that exert promoter activity during postexponential growth of Bacillus subtilis. J Bacteriol. 1983 Sep;155(3):1399–1406. doi: 10.1128/jb.155.3.1399-1406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Setlow J. K. Transformation in Micrococcus radiodurans and the ultraviolet sensitivity of its transforming DNA. Proc Natl Acad Sci U S A. 1968 Sep;61(1):176–183. doi: 10.1073/pnas.61.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean V., Claverys J. P., Vasseghi H., Sicard A. M. Rapid cloning of specific DNA fragments of Streptococcus pneumoniae by vector integration into the chromosome followed by endonucleolytic excision. Gene. 1981 Nov;15(2-3):289–293. doi: 10.1016/0378-1119(81)90139-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Pozzi G., Guild W. R. Modes of integration of heterologous plasmid DNA into the chromosome of Streptococcus pneumoniae. J Bacteriol. 1985 Mar;161(3):909–912. doi: 10.1128/jb.161.3.909-912.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Stassi D. L., Lopez P., Espinosa M., Lacks S. A. Cloning of chromosomal genes in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7028–7032. doi: 10.1073/pnas.78.11.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani A. A., Stephens R. E., D'Ambrosio S. M., Hart R. W. A sequence specific endonuclease from Micrococcus radiodurans. Biochim Biophys Acta. 1982 May 31;697(2):178–184. doi: 10.1016/0167-4781(82)90075-6. [DOI] [PubMed] [Google Scholar]