Abstract
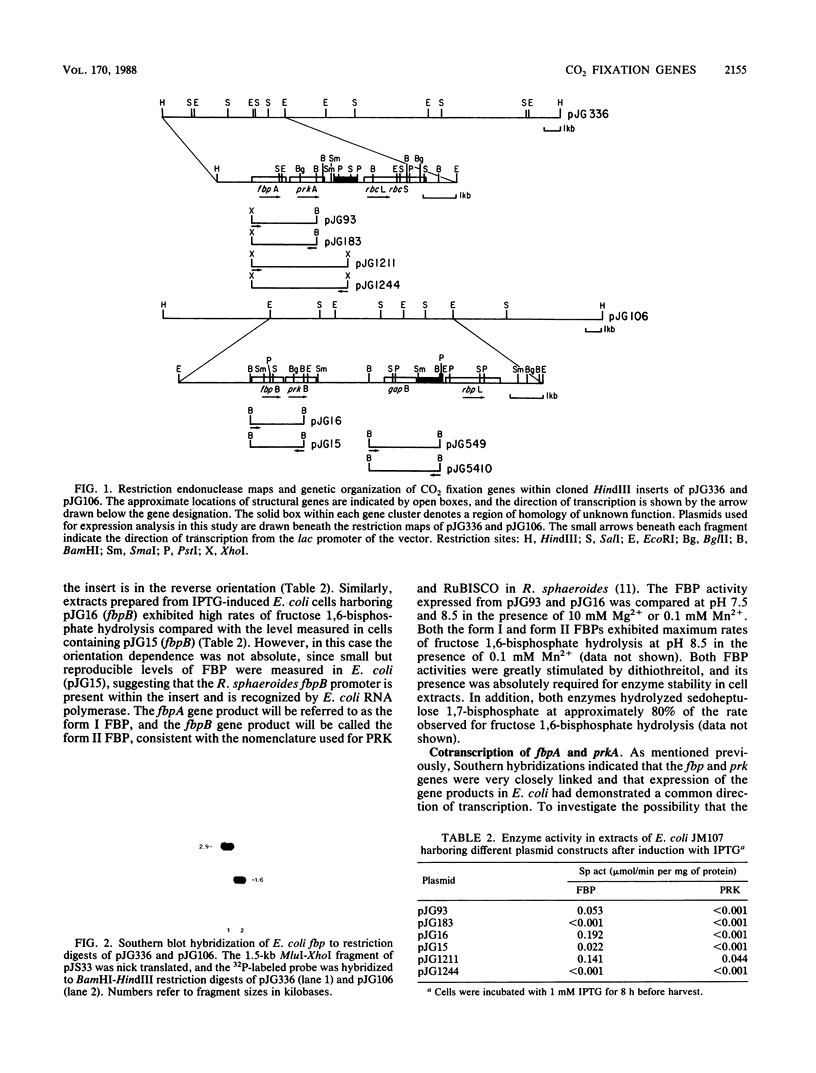
Two fructose 1,6-bisphosphatase structural genes (fbpA and fbpB) have been identified within two unlinked gene clusters that were previously shown to contain the Rhodobacter sphaeroides sequences that code for form I and form II ribulose 1,5-bisphosphate carboxylase-oxygenase and phosphoribulokinase. The fbpA and fbpB genes were localized to a region immediately upstream from the corresponding prkA and prkB sequences and were found to be transcribed in the same direction as the phosphoribulokinase and ribulose 1,5-bisphosphate carboxylase-oxygenase genes based on inducible expression of fructose 1,6-bisphosphatase activity directed by the lac promoter. A recombinant plasmid was constructed that contained the tandem fbpA and prkA genes inserted downstream from the lac promoter in plasmid pUC18. Both gene products were expressed in Escherichia coli upon induction of transcription with isopropyl beta-D-thiogalactoside, demonstrating that the two genes can be cotranscribed. A Zymomonas mobilis glyceraldehyde 3-phosphate-dehydrogenase gene (gap) hybridized to a DNA sequence located approximately 1 kilobase upstream from the form II ribulose 1,5-bisphosphate carboxylase-oxygenase gene. Although no corresponding gap sequence was found within the form I gene cluster, an additional region of homology was detected immediately upstream from the sequences that encode the form I and form II ribulose 1,5-bisphosphate carboxylase-oxygenases.
Full text
PDF



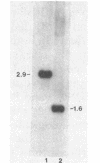
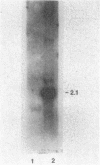
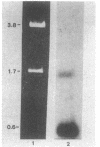
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conway T., Sewell G. W., Ingram L. O. Glyceraldehyde-3-phosphate dehydrogenase gene from Zymomonas mobilis: cloning, sequencing, and identification of promoter region. J Bacteriol. 1987 Dec;169(12):5653–5662. doi: 10.1128/jb.169.12.5653-5662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Different molecular forms of D-ribulose-1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Biol Chem. 1977 Feb 10;252(3):943–949. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Isolation of the Rhodopseudomonas sphaeroides form I ribulose 1,5-bisphosphate carboxylase/oxygenase large and small subunit genes and expression of the active hexadecameric enzyme in Escherichia coli. Gene. 1986;44(2-3):271–278. doi: 10.1016/0378-1119(86)90191-5. [DOI] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Organization of phosphoribulokinase and ribulose bisphosphate carboxylase/oxygenase genes in Rhodopseudomonas (Rhodobacter) sphaeroides. J Bacteriol. 1987 Aug;169(8):3685–3690. doi: 10.1128/jb.169.8.3685-3690.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. L., Kaplan S. Cloning of the gene for phosphoribulokinase activity from Rhodobacter sphaeroides and its expression in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3669–3678. doi: 10.1128/jb.169.8.3669-3678.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint I. R., Morris I., Fuller R. C. Purification of a complex of alkaline fructose 1,6-bisphosphatase and phosphoribulokinase from Rhodospirillum rubrum. J Biol Chem. 1972 Aug 10;247(15):4833–4838. [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. In vivo regulation of form I ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1987 Apr;254(1):290–303. doi: 10.1016/0003-9861(87)90105-6. [DOI] [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. Independent regulation of synthesis of form I and form II ribulose bisphosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J Bacteriol. 1986 Feb;165(2):620–624. doi: 10.1128/jb.165.2.620-624.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Muller E. D., Chory J., Kaplan S. Cloning and characterization of the gene product of the form II ribulose-1,5-bisphosphate carboxylase gene of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jan;161(1):469–472. doi: 10.1128/jb.161.1.469-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivey R. G., Jr, Tabita F. R. Cloning and expression in Escherichia coli of the form II ribulose 1,5-bisphosphate carboxylase/oxygenase gene from Rhodopseudomonas sphaeroides. Gene. 1984 Nov;31(1-3):91–101. doi: 10.1016/0378-1119(84)90198-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sedivy J. M., Daldal F., Fraenkel D. G. Fructose bisphosphatase of Escherichia coli: cloning of the structural gene (fbp) and preparation of a chromosomal deletion. J Bacteriol. 1984 Jun;158(3):1048–1053. doi: 10.1128/jb.158.3.1048-1053.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springgate C. F., Stachow C. S. Fructose 1,6-diphosphatase from Rhodopseudomonas palustris. I. Purification and properties. Arch Biochem Biophys. 1972 Sep;152(1):1–12. doi: 10.1016/0003-9861(72)90186-5. [DOI] [PubMed] [Google Scholar]
- Tabita F. R. Pyridine nucleotide control and subunit structure of phosphoribulokinase from photosynthetic bacteria. J Bacteriol. 1980 Sep;143(3):1275–1280. doi: 10.1128/jb.143.3.1275-1280.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase. Eur J Biochem. 1976 Nov 15;70(2):361–367. doi: 10.1111/j.1432-1033.1976.tb11025.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Purification and properties of spinach leaf cytoplasmic fructose-1,6-bisphosphatase. J Biol Chem. 1978 Sep 10;253(17):5952–5956. [PubMed] [Google Scholar]