Abstract
In vertebrates, spinal commissural axons project along a transverse path toward and across the floor plate (FP). Post-crossing commissural axons alter their responsiveness to FP-associated guidance cues and turn to project longitudinally in a fasciculated manner prior to extending away from the midline. The upregulation of the neural cell adhesion molecule L1 on crossed commissural axon segments has been proposed to facilitate pathfinding on the contralateral side of the FP. To explore this possibility in vivo, we used Math1 regulatory sequences to target L1 to commissural axons before they cross the ventral midline. L1 mis-expression did not alter the distribution of commissural axon-associated markers or the ventral extension of commissural axons toward the midline. However, commissural axons often stalled or inappropriately projected into the longitudinal plane at the ipsilateral FP margin. These observations suggest that L1-mediated pathfinding decisions are normally delayed until axons have crossed the ventral midline (VM).
Keywords: Math1, floor plate, altered responsiveness, commissural axon, L1, fasciculation
Introduction
In bilaterally symmetric organisms, large numbers of neurons extend axons that project across the central nervous system (CNS) midline and then abruptly alter their trajectory (Kaprielian et al., 2001; Dickson, 2002; Williams et al., 2004). A key question is how, when confronted with the same set of cues on either side of the midline, do outgrowing commissural axons/growth cones apparently ignore some of these cues on the ipsilateral side of the midline, but then respond to them when they emerge on the contralateral side?
Dynamic changes in the receptor systems that interpret bilaterally distributed axon guidance cues appear to underlie the altered responsiveness of commissural axons. In both vertebrates (Dodd et al., 1988; Imondi et al., 2000; Brittis et al., 2002; Long et al., 2004; Sabatier et al., 2004) and invertebrates (Kidd et al., 1998; Rajagopalan et al., 2000), stereotypic changes in the spatial distribution of guidance receptors occur along the different segments of a commissural axon’s trajectory. For instance, in the developing mammalian spinal cord, TAG-1 (Dodd et al., 1988) and Rig-1 (Sabatier et al., 2004) are expressed at high levels on commissural axons as they project ventrally toward and across the FP, but are downregulated on decussated segments of these axons. Conversely, L1 (Dodd et al., 1988), EphB1 (Imondi et al., 2000), EphA2 (Brittis et al., 2002) and Robo1/2 (Long et al., 2004) are thought to be upregulated on commissural axons as they exit the FP and turn into the longitudinal axis. NrCAM, on the other hand, is preferentially expressed on axon segments that are in transit across the midline (Lustig et al., 2001).
In addition to changes in receptor expression per se, in vitro data suggest that alterations in the sensitivity of axon guidance receptors that respond to attractive (Shirasaki et al., 1998) and repulsive (Zou et al., 2000; Stein and Tessier-Lavigne, 2001; Sabatier et al., 2004; Dickson and Gilestro, 2006) cues may account, at least in part, for differences in pathfinding behavior observed on each side of the midline. Thus, several converging lines of data seem to indicate that the responsiveness of commissural axons is influenced by concomitant changes in the deployment and/or sensitivity of receptor systems that act at different points along their trajectory.
To critically evaluate several important aspects of this model in vivo, we developed a system in which axon guidance receptors could be selectively manipulated on a particular subset of commissural axons in an otherwise wild-type animal. In the embryonic mouse spinal cord, the basic helix-loop-helix (bHLH) transcription factor, Math1, is selectively expressed in a unique progenitor population that gives rise to dI1 neurons, which are defined by their expression of the Lim homeodomain transcription factors, Lhx2 (LH2A) and Lhx9 (LH2B), as well as Brn3A and BarH1 (see Caspary and Anderson, 2003; Helms and Johnson, 2003). Consistent with the identification of dI1 as a commissural neuron population, an enhancer element derived from the Math1 gene drives reporter gene expression in the spinal cord of transgenic mice within cells that settle in the deep dorsal horn and that extend axons across the FP (Helms and Johnson, 1998; Helms et al., 2000). Given these observations, we reasoned that it might be possible to use the Math1 enhancer to mis-express cell surface proteins in a genetically defined subset of commissural neurons. This would, in principle, provide a tractable in vivo system within which to examine the function of individual guidance receptor systems and their potential interplay during commissural axon pathfinding.
As a first step toward this goal, we examined the role of L1 in mediating the pathfinding behavior of commissural axons. L1 is a cell surface molecule of the immunoglobulin superfamily (IgCAM; Hortsch, 1996; Brummendorf et al., 1998) that regulates neurite outgrowth and axon fasciculation through both homophilic and heterophilic adhesion (Grumet and Sakurai, 1996; Hortsch, 1996; Itoh et al., 2004; Maness and Schachner, 2007). The apparent sharp upregulation of L1 on crossed commissural axons has been thought to play an important role in commissural axon pathfinding on the contralateral side of the midline, where newly decussated commissural axons turn rostrally and project alongside the FP. Although purely speculative, the delay in L1 expression has been proposed to forestall growth in the longitudinal axis until commissural axons have crossed the ventral midline, where they can engage other axons and lateral FP cells that express L1 and/or L1-binding proteins. To test this hypothesis, we used regulatory sequences derived from the Math1 gene to mis-express L1 on pre-crossing axons that emanate from dI1 commissural neurons. In Math1/L1 transgenic mice, many commissural axons exhibited pathfinding errors that may arise from premature L1-mediated contact-dependent interactions at the ventral midline. These findings establish the feasibility of manipulating receptor expression along spinal commissural axons in vivo, an approach that we are currently using to assess the pathfinding roles of other cell surface proteins that display spatially restricted, axon segment-specific expression patterns.
Results
A transgenic approach to the study of altered responsiveness at the spinal cord midline
During their migration toward and across the FP, axons extending from dorsal commissural neurons express TAG-1, but not L1, protein. As these axons exit the FP and project longitudinally, TAG-1 expression is maintained on uncrossed segments and L1 appears to be concomitantly upregulated and maintained on decussated segments extending on the contralateral side of the spinal cord (Dodd et al., 1988; Imondi et al., 2000). However, it is important to note in this regard, that the upregulation of L1 on post-crossing segments of spinal commissural axons has not been definitively established. We have extended these findings by showing that L1 mRNA is not expressed by dorsal commissural neurons when their axons are projecting towards the FP (see Imondi et al., 2000), but is expressed at high levels by ventrally located motor neurons (Supp. Fig. 1A). At E12 and E13, after many commissural axons have crossed through the FP (Imondi and Kaprielian, 2001), L1 mRNA is expressed in a broad ventral domain that extends dorsolaterally (Supp. Fig. 1B, C) into a region occupied by ipsilaterally- and contralaterally-projecting interneurons (see Imondi et al., 2000).
Figure 1. Math1 regulatory sequences drive transgene expression in commissural neurons/axons.
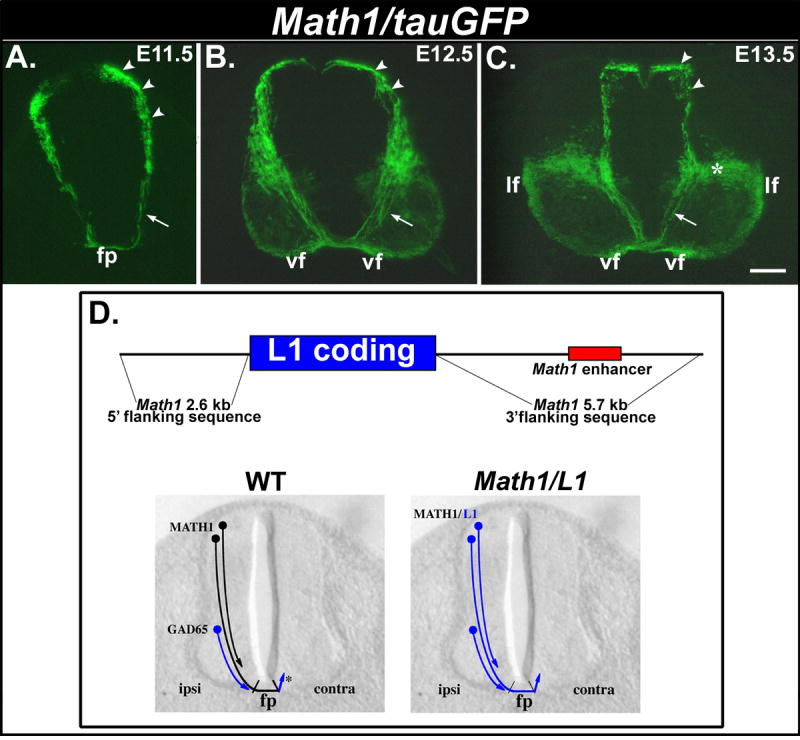
(A-C) Transverse cryosections obtained from E11.5 (A), E12.5 (B) and E13.5 (C) Math1/tauGFP embryos. (A, B) At E11.5 and E12.5, tauGFP is expressed by dorsally-located cell bodies of Math1 progenitors, the commissural neurons derived from these progenitors (arrowheads), and the corresponding axons (arrows) that extend ventrally toward and across the floor plate (fp) and assemble into the longitudinally projecting ventral funiculus (vf). (C) Expression of tauGFP in commissural neurons/axons persists in E13.5 embryos. A large, ventrally located population of neurons (asterisk) that appears to extend axons directly into the lateral funiculus (lf) also expresses the transgene at this age. (D, top panel) Construct used to generate Math1/L1 transgenic mice. Genomic sequences flanking the Math1 coding region (black line), including the Math1 enhancer (red), were used to flank the mouse L1 coding sequence (blue). (D, lower panels) In wild-type (WT) embryos, L1 is expressed on axons emanating from Math1-positive dorsal commissural axons only after they cross through the FP (asterisk). A more ventral population of GAD-65-positive commissural neurons extends L1-positive axons prior to reaching the FP. In Math1/L1 mice, the L1 transgene should be expressed on pre-crossing segments of axons emanating from dorsal commissural neurons. Scale bar in (C), 100 μm (A-C). ipsi, ipsilateral; contra, contralateral.
The presumed upregulation of L1 protein on post-crossing commissural axons and mRNA in the corresponding neuronal cell bodies at E12 and E13 suggests a role for L1 in modulating commissural axon pathfinding on the contralateral side of the ventral midline. Specifically, L1-mediated interactions between newly decussated commissural axons and other axons or lateral FP cells might facilitate their growth transition into the longitudinal axis.
To explore this possibility in vivo, we first assessed the ability of Math1 regulatory sequences to drive reporter protein expression to commissural axons prior to midline crossing. In transgenic mice that express lacZ under the control of Math1 regulatory sequences, β-galactosidase is expressed within dorsally located, dI1 commissural neurons and their axons (Helms and Johnson, 1998). To visualize these particular commissural projections at higher resolution, we used Math1 regulatory sequences to drive the expression of tauGFP in this same population of commissural neurons.
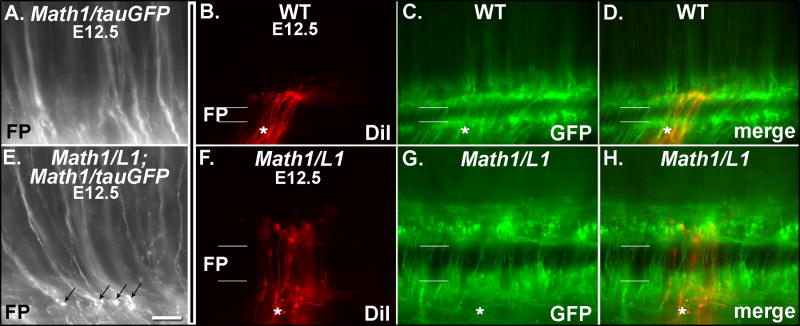
In E11.5 Math1/tauGFP embryos, GFP is expressed in a dorsolateral region of the spinal cord that contains the cell bodies of Math1 progenitors and dI1 commissural neurons that arise from these progenitors. GFP is also expressed along the pre-crossing segments of commissural axons that extend from dI1 neurons and project ventrally toward and across the FP (Fig. 1A). Expression of tauGFP by dorsal commissural neurons/axons persists at E12.5 and E13.5; at these ages commissural axons have extended for considerable distances on the contralateral side of the spinal cord, where they assemble into longitudinally-oriented funiculi (Fig. 1B, C; see Imondi and Kaprielian, 2001). At E13.5, a conspicuous domain of GFP expression is also observed in a more ventral region of the spinal cord (white asterisk in Fig. 1C). This domain encompasses both dI1 commissural neurons that have settled in the deep dorsal horn, as well as a subpopulation of dI1 neurons that extend their axons ipsilaterally into the lateral funiculi (Fig. 1C; see Silos-Santiago and Snider, 1994). These observations demonstrate that Math1 flanking sequences are capable of directing reporter gene expression to dorsal commissural neurons and their axons before, during, and after they cross the ventral midline. We therefore used Math1 flanking sequences to drive the expression of L1 on dI1 neurons in Math1/L1 transgenic mice (Fig. 1D).
L1 is mis-expressed on pre-crossing segments of commissural axons in Math1/L1 transgenic mouse embryos
In E11.5 wild-type embryos, L1 is expressed by sensory and motor axons, as well as decussated segments of commissural axons located within ventral and ventrolateral funiculi (see Fig. 2A). In addition, L1 is expressed on both pre-and post-crossing segments of axons emanating from a late-developing ventral population of GABAergic commissural neurons (see Tran and Phelps, 2000; Figs. 1D, 2A, 5G, H). Importantly, L1 is not detected on dorsal spinal commissural neurons or the pre-crossing segments of their axons in wild-type embryos (see Fig. 1D and Fig. 2A). In contrast, within E11.5 Math1/L1 transgenic embryos, L1 was mis-expressed on the cell bodies of dorsally located Math1 progenitors and dI1 commissural neurons, as well as on the pre-crossing commissural axons that extend from these neurons and appropriately project towards the ventral midline (Fig. 2B, D). Importantly, ectopic L1 expression is present along the full extent of pre-crossing axons, including the more proximal, dorsally located portions and the more distal, ventrally located segments (Fig. 2). Consistent with these axons emanating from dI1 commissural axons (Helms and Johnson, 1998), TAG-1 (Dodd et al., 1988) is co-expressed with L1 on GFP-positive, pre-crossing commissural axons in cryosections derived from Math1/L1 embryos (Fig. 3).
Figure 2. L1 is mis-expressed on pre-crossing segments of commissural axons in Math1/L1 mice.
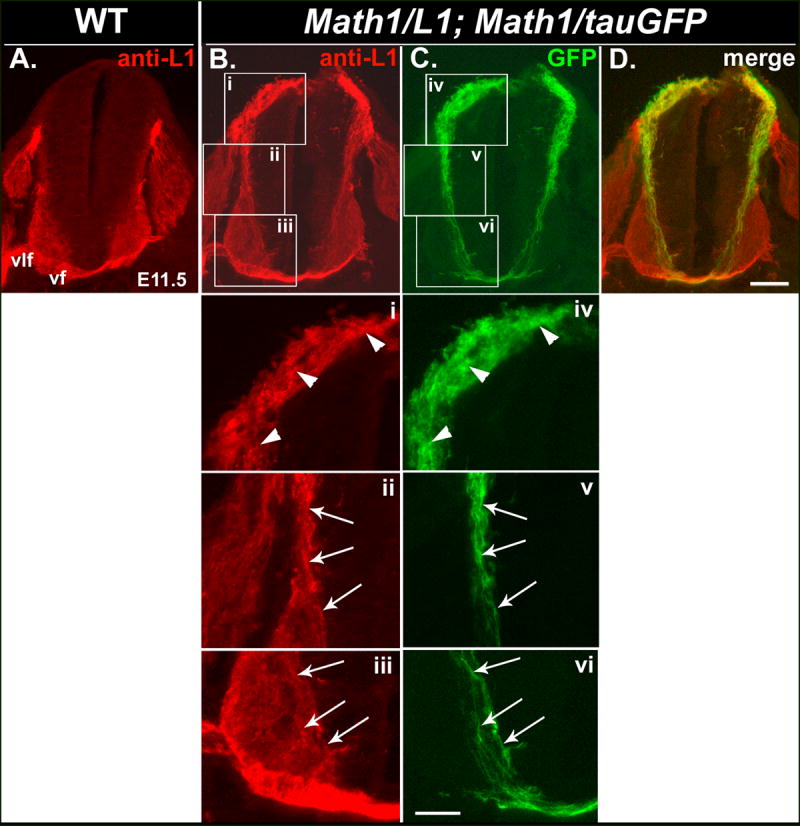
(A-D) Transverse cryosections obtained from an E11.5 wild-type embryo (A) or an E11.5 embryo derived from a cross between Math1/L1 and Math1/tauGFP mice (B-D) that were labeled with anti-L1. In Math1/L1;Math1/tauGFP (B-D), but not in wild-type (A), embryos, anti-L1 labels dorsally located cell bodies of Math1 progenitors, the commissural neurons derived from these progenitors, and the corresponding axons of these cells, which project ventrally toward the FP and express the tauGFP transgene (C, D). Panel (D) represents a merge of panels (B) and (C). Ventral funiculus (vf), ventrolateral funiculus (vlf). Scale bar in (D), 100 μm (A-D). (i-vi) Higher magnification views of boxed regions in panels (B) and (C). Arrowheads (i, iv) indicate L1- and GFP-expressing cell bodies of Math1 progenitors and post-mitotic dI1 commissural neurons, as well as the most proximal portions of their pre-crossing axons. Arrows identify pre-crossing segments of L1- and GFP-expressing commissural axons in an intermediate (ii, v) and a ventral (iii, vi) region of the spinal cord. Scale bar in (vi), 50 μm (i-vi).
Figure 5. L1 mis-expression does not affect the ventral extension of commissural axons or the distribution of commissural axon markers.
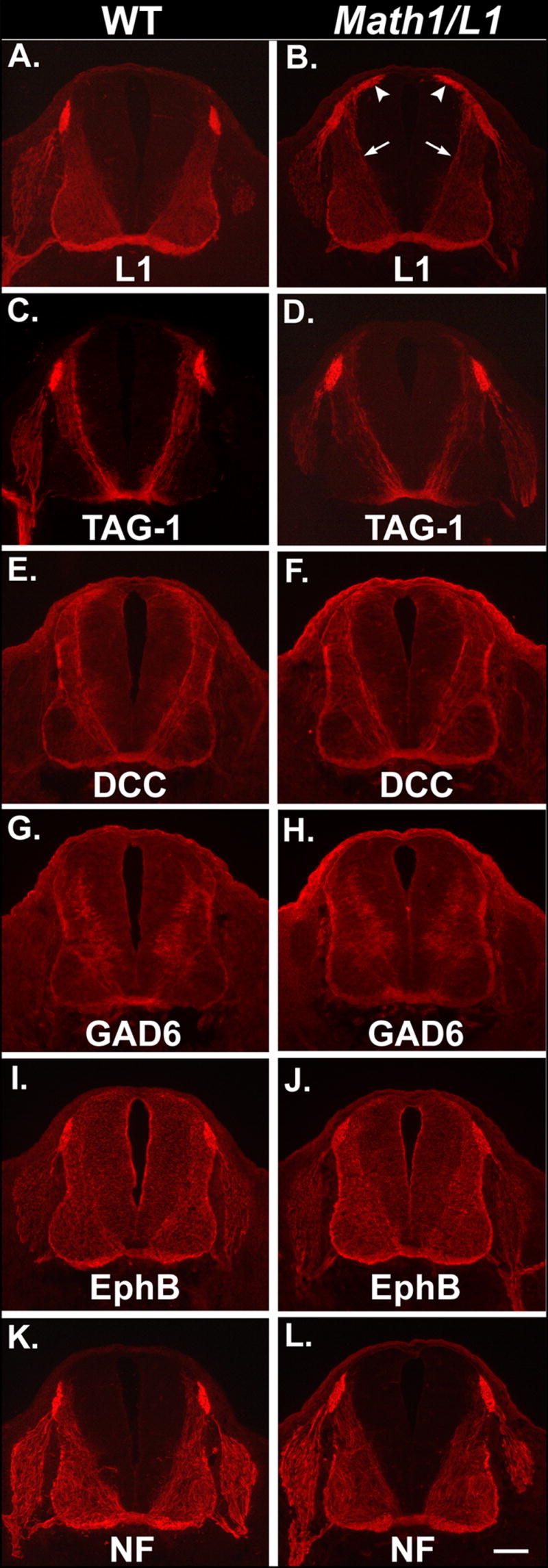
(A-L) Semi-serial transverse cryosections obtained from E12.5 wild-type (left column) or Math1/L1 embryos (right column) that were labeled with a variety of commissural axon markers. In Math1/L1 embryos, L1 is mis-expressed on dorsally located cell bodies of Math1 progenitors and the commissural neurons derived from these progenitors (white arrowheads), as well as on the corresponding axons (arrows) that extend ventrally toward and across the floor plate (B). The distribution of other commissural axon markers is unaffected in Math1/L1 embryos (D, F, H, J, L). Scale bar in (L), 100 μm (A-L).
Figure 3. The distribution of TAG-1 overlaps with the expression of tauGFP and L1 in Math1/L1; Math1/tauGFP and Math1/L1 mice, respectively.
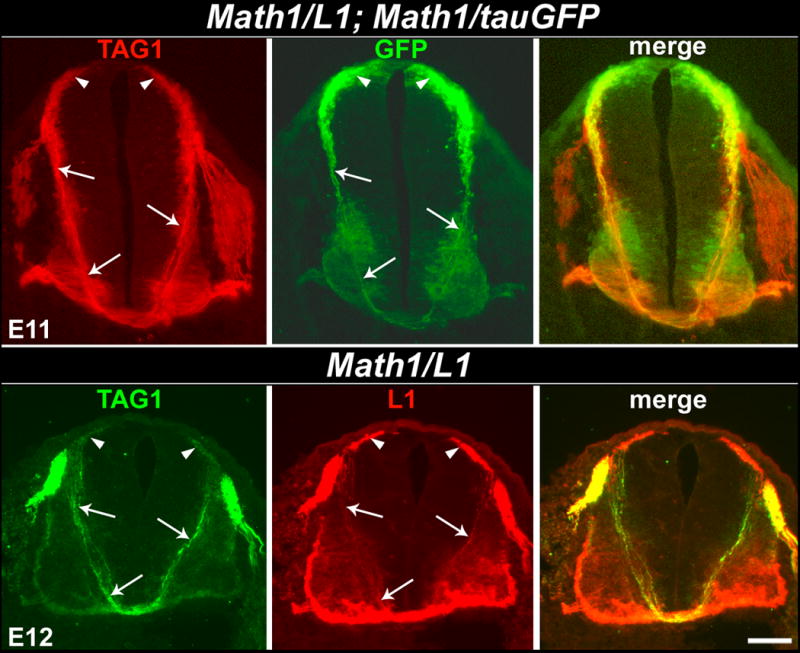
(Top panels) Transverse cryosection obtained from an E11 Math1/L1; Math1/tauGFP embryo labeled with anti-TAG-1. TAG-1 (left) and GFP (middle) expression overlap on the dorsally located cells bodies of Math1 progenitors and post-mitotic commissural neurons (arrowheads), as well as on the pre-crossing segments of their commissural axons (arrows). (Bottom panels) Transverse cryosection obtained from an E12 Math1/L1 embryo labeled with anti-TAG-1 and anti-L1. TAG-1 (left) and L1 (middle) expression overlap on the dorsally located cells bodies of Math1 progenitors and post-mitotic commissural neurons (arrowheads), as well as on the pre-crossing segments of their commissural axons (arrows). In each case, the right panel represents a merge of the left and middle panels. Scale bar in lower right panel, 100 μm (all panels).
In E10.5 Math1/L1 embryos, mis-expression of L1 is observed in Math1 progenitors, as well as in dI1 neurons and on the pre-crossing segments of their axons that project along the lateral margins of the spinal cord, but that have not yet reached the ventral midline (Fig. 4B). By E12.5, L1 mis-expression persists on the cell bodies of Math1 progenitors and dI1 neurons, and is present along the entire length of their pre-crossing axonal segments, which have projected to the FP at this embryonic stage (Fig. 4D).
Figure 4. Mis-expression of L1 is detectable at E10.5 and E12.5 in Math1/L1 mice.
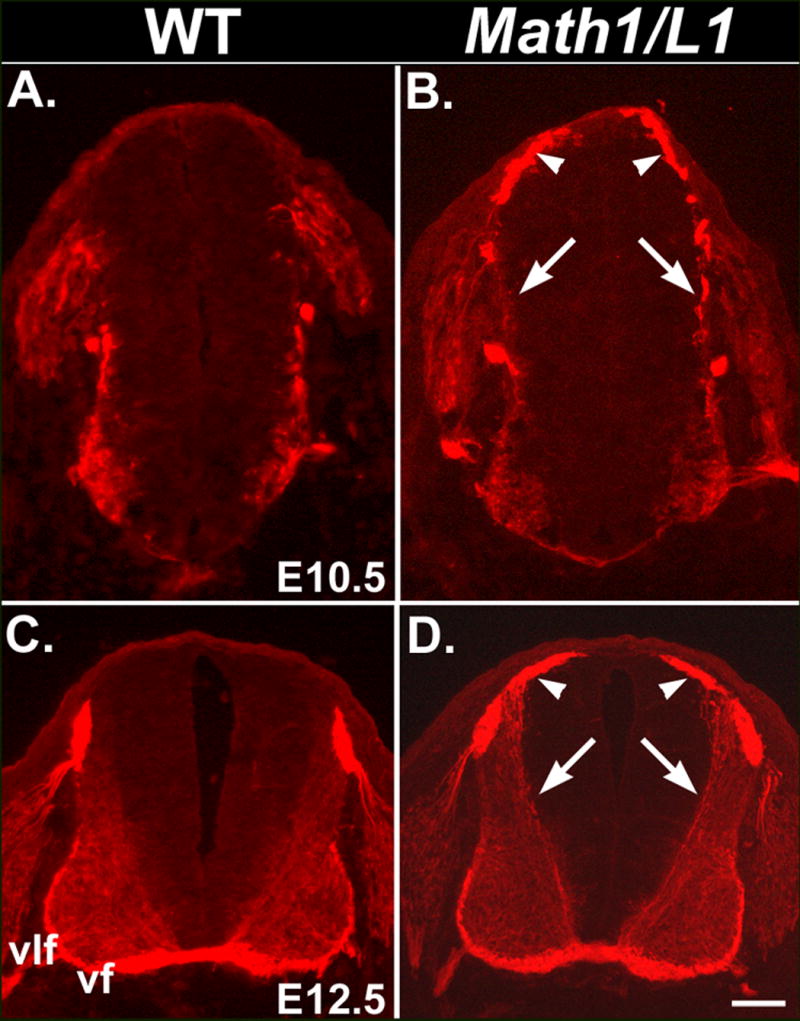
(A-D) Transverse cryosections were obtained from E10.5 and E12.5 wild-type (A, C) or Math1/L1 (B, D) embryos and labeled with anti-L1. In Math1/L1 embryos, ectopic L1 expression on the cell bodies of Math1 progenitors and the commissural neurons derived from these progenitors (arrowheads), as well as on their ventrally projecting axons (arrows), is detected at both E10.5 (B) and E12.5 (D). The punctate anti-L1 labeling of axons at E10.5 (arrows in B) reflects the relatively poor morphology characteristic of cryosections obtained from these young embryos. Ventral funiculus (vf), ventrolateral funiculus (vlf). Scale bar in (D), 100 μm (C, D); 65 μm (A, B).
Mis-expression of L1 does not affect the ventral extension of commissural axons toward the ventral midline or the distribution of commissural axon-associated proteins
To independently determine whether L1 mis-expression (see Fig. 5B) impaired the ability of commissural axons to extend ventrally toward the FP, we compared the expression of TAG-1 and DCC (Keino-Masu et al., 1996), two well-established markers of pre-crossing commissural axons, in wild-type and transgenic littermates. In E12.5 Math1/L1 embryos, the ventral extension of TAG-1- and DCC-positive axons was indistinguishable from that observed in wild- type embryos (Fig. 5C-F). Similarly, mis-expression of L1 does not alter the ventrally directed growth of axons emanating from a relatively late-developing ventral population of GAD65-positive commissural neurons (Fig. 5G-H). In addition, the distribution of EphB and neurofilament-associated proteins, which are preferentially expressed on decussated segments of commissural axons, is unaffected in Math1/L1 embryos (Fig. 5I-L).
Commissural axons exhibit midline pathfinding defects in Math/L1 mice
To assess commissural axon pathfinding at the ventral midline in Math1/L1 mice, we initially used unilateral anterograde DiI labeling (Fig. 6, Schematic) to visualize small groups of axons that project to the contralateral side of the spinal cord. Specifically, we iontophoretically applied minute crystals of DiI into dorsal regions of open-book spinal cord preparations obtained from E12.5 wild-type and Math1/L1 embryos (Imondi and Kaprielian, 2001; Kadison and Kaprielian, 2004). For each DiI application, a given group of labeled axons is likely to represent a mixture of L1-mis-expressing and wild-type commissural axons. Consistent with previous observations (Bovolenta and Dodd, 1990; Imondi and Kaprielian, 2001), commissural axons projected directly through the FP in a relatively straight and well-organized manner before turning into the longitudinal plane at the contralateral FP boundary in many wild type embryos (Fig. 6A, D). In contrast, DiI labeled axons in Math1/L1 embryos were frequently observed to stall, pile-up, or inappropriately turn into the longitudinal plane at the ipsilateral margin of the FP (Figs. 6B, C, E). Stalling was also occasionally observed at the contralateral margin of the FP in open-book preparations derived from Math1/L1 embryos (Fig. 6F). Higher magnification/resolution views of DiI-labeled open-book preparations obtained from E12.5 Math1/L1 embryos confirm the presence of swollen or bulbous growth cones on the ipsilateral side of the FP (Fig. 7A, B). This morphology has previously been associated with stalling commissural axons in rodent spinal cord preparations (see Zou et al., 2000; Long et al., 2004; Shirasaki and Murakami, 2001). In contrast, more streamlined, wild type-like growth cones tip those axons that inappropriately turn into, and project along, the longitudinal plane on the ipsilateral side of the FP in these embryos (Fig. 7A).
Figure 6. Mis-expression of L1 results in commissural axon projection errors in the immediate vicinity of the FP.
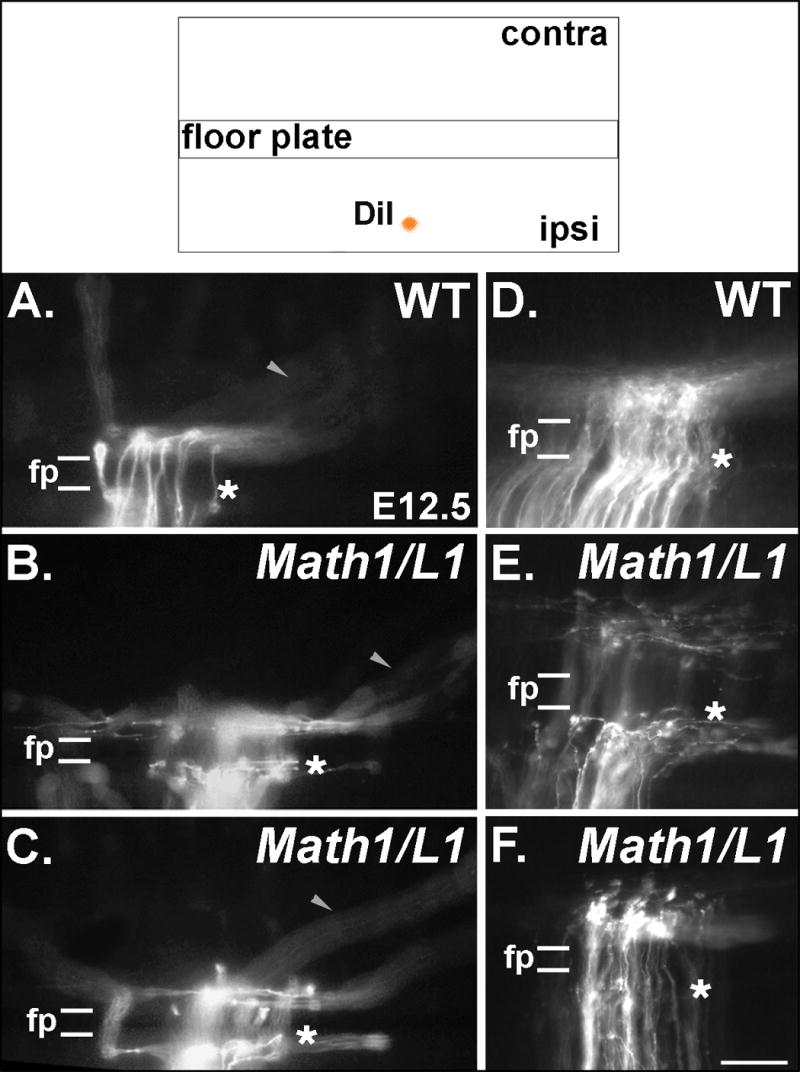
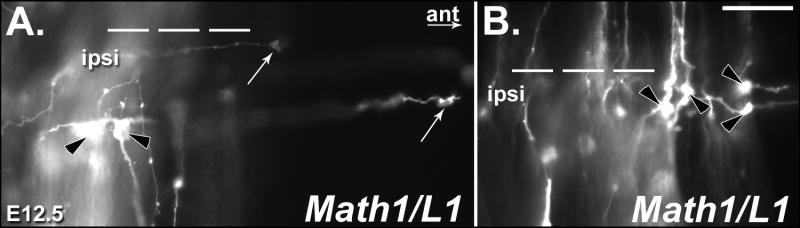
(Top) The pathfinding behavior of commissural axons was visualized through the application of DiI into a dorsal region of fixed, open-book spinal cord preparations taken from E12.5 embryos. (A-F) Photomicrographs of DiI-labeled axons in open book preparations derived from wild type (WT) and Math1/L1 embryos. (A, D) In WT embryos, commissural axons project directly through the FP in a well-organized fashion before turning in a predominantly rostral direction on the contralateral side of the spinal cord. (B, C, E) In Math1/L1 embryos, commissural axons “pile-up” and stall at the ipsilateral margin of the FP, or project along this boundary before ultimately crossing the FP. The arrowheads in A, B and C denote out of focus axons that are projecting along appropriately shaped contralateral trajectories in WT and Math1/L1 embryos. (F) On rare occasions, some commissural axons were observed to stall at the contralateral margin of the FP. In A-F, asterisks demarcate the position of the ipsilateral FP margin. In each panel, rostral is to the right. Scale bar in (F), 95 μm (A-C); 50 μm (D-F).
Figure 7. Many of the commissural axon-associated growth cones in Math1/L1 embryos display a swollen/bulbous morphology typically associated with a stalling behavior.
(A, B) Photomicrographs of DiI-labeled axons in open book preparations derived from E12.5 Math1/L1 embryos. (A) This particular open-book preparation contains examples of both swollen/bulbous, stalled growth cones (black arrowheads) and commissural axons tipped by wild type-like growth cones (white arrows) that inappropriately turn into the longitudinal plane on the ipsilateral side of the FP. (B) In this open-book preparation, there are at least four examples of presumably stalled growth cones (black arrowheads). In each panel, the dashed lines demarcate the position of the FP. Ipsi, ipsilateral; ant, anterior direction. Scale bar in (B), 25 μm (A, B).
To quantify these phenotypes, wild type and Math1/L1 embryos were classified as either aberrant or unaffected, depending on whether or not one or more of the open-book preparations derived from a given embryo contained at least two DiI-labeled axons that either stalled at the ipsilateral (or contralateral, in rare cases) margin of the FP or that inappropriately turned into the longitudinal plane prior to crossing the FP. Of the 31 wild-type embryos examined, 9 were classified as aberrant and 22 exhibited an unaffected phenotype. On the other hand, of the 20 Math1/L1 embryos examined, 13 exhibited the aberrant phenotype and 7 were classified as wild type (Fig. 8). The finding that many commissural axons in Math1/L1 embryos appear to stall, or inappropriately turn into the longitudinal plane, at the ipsilateral margin of the FP is likely to reflect their ability to prematurely interact with other axons and/or lateral FP cells. Despite these errors, many commissural axons ultimately crossed through the FP and assumed the appropriate contralateral trajectory (data not shown).
Figure 8. Quantification of pathfinding errors in wild-type (WT) and Math1/L1 embryos.
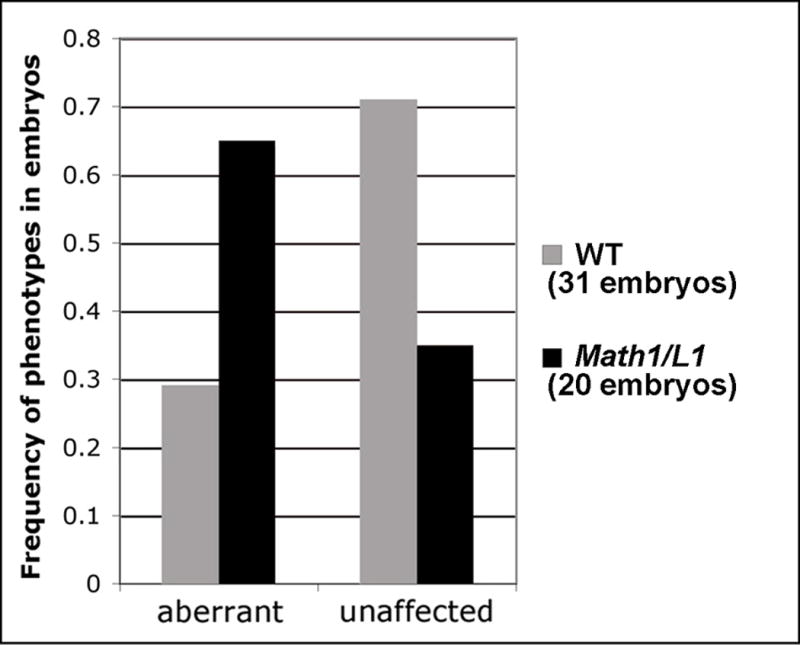
Frequency histogram summarizing the phenotypes (unaffected and aberrant) observed in Math1/L1 versus WT embryos (see text for scoring criteria and details).
In an attempt to determine whether the axons that exhibit pathfinding errors at the ipsilateral margin of the FP in Math1/L1 embryos mis-express L1, we examined GFP expression in open-book preparations derived from E12.5 wild type and Math1/L1 embryos in a Math1/tauGFP background. These analyses identified several examples of what appear to be swollen/bulbous (i.e., stalling), GFP-labeled growth cones in Math1/L1;Math1/tauGFP (Fig. 9E), but not in Math1/tauGFP (Fig. 9A), embryos.
Figure 9. GFP-labeled commissural axons exhibit pathfinding defects in Math1/L1;Math/tauGFP (Math1/L1) embryos.
(A, E) Photomicrographs of GFP-labeled commissural axons in open book preparations derived from an E12.5 Math1/tauGFP (A) and a Math1/L1;Math1/tauGFP (E) embryo. Several examples of stalled growth cones (black arrows in E) are observed in the open-book preparation derived from the Math1/L1;Math1/tauGFP, but not the Math1/tauGFP, embryo. The FP is located at the bottom of each panel. (B-D, F-H). Photomicrographs of DiI- or GFP-labeled commissural axons in open book preparations derived from a Math1/tauGFP (WT; B-D) and a Math1/L1;Math1/tauGFP (Math1/L1; F-H) embryo. (B, F) DiI labeling. (C, G) GFP expression. (D, H) Merge of DiI labeling and GFP expression. In B-D and F-H, the asterisk demarcates the ipsilateral side of the FP. In each panel, rostral is to the right. Scale bar in (E), 25 μm (A, E); 45 μm (B-D, F-H).
We also assessed the potential overlap between DiI- and GFP-labeled axons in some of these preparations. Consistent with the findings described above, DiI labeling identified axons that stalled or inappropriately turned into the longitudinal plane at the ipsilateral margin of the FP in Math/L1 (Fig. 9F), but not in wild type (Math1/tauGFP; Fig. 9B) embryos. Despite the focal applications of DiI, it was not possible to accurately determine the proportion of mis-projecting DiI-labeled axons that also expressed GFP in open book preparations derived from both wild type and Math/L1;Math1tauGFP embryos. This was largely due to intense GFP expression in axons within the ventral commissure and ventral funiculi, coupled with the interdigitation, within and at the boundaries of the FP, of commissural axons emanating from cell bodies on either side of the spinal cord. Nevertheless, at least a subset of the small number of DiI labeled axons that commit pathfinding errors in these preparations appeared to also express GFP (see Fig. 9G, H). Taken together, these observations suggest that both L1-positive and L1-negative commissural axons inappropriately stall at the ipsilateral margin of the FP in Math1/L1 mice.
Discussion
In the mammalian spinal cord, L1 protein is likely to be sharply upregulated on decussated commissural axons as they turn to initiate growth in the longitudinal axis. Despite the lack of direct evidence supporting the “upregulation” of L1 on post-crossing segments of dorsal commissural axons, L1 is clearly absent from the pre-crossing segments of these axons and is expressed at high levels in the decussated commissural axon-rich marginal zone. While sequences present within the 3’UTR of L1 mRNA may control the spatial regulation of L1 protein (see Brittis et al., 2002), the functional significance of the resulting axon segment-specific expression pattern is not known. In the present study, we used a transgenic strategy to disrupt the spatial segregation of L1 on commissural axons. This was achieved through the use of a Math1 transgene construct that drives heterologous gene expression in a subset of dorsal commissural neurons during the period when they extend axons toward and across the FP. In Math1/L1 mice, L1 is mis-expressed on pre-crossing segments of these commissural axons. Although L1 mis-expression did not affect the extension of commissural axons toward the ventral midline or the distribution of commissural axon-associated proteins, clear projection errors were observed in the immediate vicinity of the FP.
In Math1/L1 embryos, intense ectopic L1 expression is present on the cell bodies of Math1 progenitors and post-mitotic dI1 commissural neurons, as well as on the most proximal, pre-crossing segments of commissural axons that emerge from these dorsally located cells. While we have shown that L1 is mis-expressed along the entire length of pre-crossing commissural axons, the level of L1 expression appears somewhat diminished on ventral segments of these axons. This apparent reduction of ectopic L1 expression mimics the decline in GFP expression observed in more ventral segments of pre-crossing axons in Math1tauGFP embryos. We believe that the reduced intensity of the mis-expressed proteins in the two transgenic lines most likely reflects the defasciculation of pre-crossing commissural axons that occurs in more ventral regions of the spinal cord as these axons approach the floor plate (see below). However, it is formally possible that a block in protein translation (see Brittis et al., 2002) may contribute to the apparent reduction of both ectopic L1 and tauGFP expression. Similarly, a temporal block in translation may account, at least in part, for our observation that by E14, mis-expression of L1 and GFP is no longer detectable in the spinal cords (either on Math1 progenitors, dI1 neurons, or pre-/post-crossing segments of the corresponding axons) of Math1/L1 and Math1/tauGFP mice, respectively (data not shown).
The dramatic segregation of L1 to post-crossing segments of commissural axons that undergo an abrupt transition from transverse to longitudinal growth may result from a prior interaction between pre-crossing commissural axons and FP cells (Shirasaki and Murakami, 2001; but also see Kadison et al., 2006). Regardless of the mechanism underlying this spatial segregation, the restriction of L1 to decussated commissural axons was originally proposed to confer upon these axons responsiveness to specific guidance cues only on the contralateral side of the spinal cord (Dodd et al., 1988). In the context of this model, L1 expression on pre-crossing commissural axons should enable these axons to prematurely respond to cues on the ipsilateral side of the midline (see Shirasaki and Murakami, 2001) and, consequently, interfere with their navigation in the immediate vicinity of the FP. The recent availability of the Math1 cassette to drive heterologous gene expression in proliferating and newly differentiated commissural neuron populations provided a tractable in vivo system within which to test this prediction. The power of this approach rests largely on the ability of Math1 regulatory sequences to selectively initiate L1 expression in a specific subset of commissural neurons before their axons have approached and made contact with the FP.
The ability of commissural axons to migrate normally toward the ventral midline in Math1/L1 embryos was not unexpected given the presumed role of L1 in mediating pathfinding events within the immediate vicinity of the FP. On the other hand, a significant number of commissural axons displayed projection errors at the ipsilateral border of this structure in Math1/L1 embryos, where they exhibited an apparent inability to grow directly through the FP and, instead, stalled or inappropriately projected into the longitudinal plane at the ipsilateral margin of the FP. These observations suggest that mis-expression of L1 on pre-crossing segments of dorsal commissural axons enables them to prematurely respond to one or more navigational cues on the ipsilateral side of the FP. This is consistent with the observation that changes in commissural axons induced by FP contact (e.g., presumed upregulation of L1), activates a navigational program that allows these axons to make the transition from circumferential to longitudinal growth only after crossing the FP (Shirasaki and Murakami, 2001). We have recently observed that most GFP-labeled axons markedly de-fasciculate immediately prior to entering the FP in Math1tauGFP mouse embryos (data not shown). Accordingly, this reduced fasciculation may facilitate the premature turn into the longitudinal plane by enabling some commissural axons to engage in L1-mediated interactions with other longitudinally projecting axons on the ipsilateral margin of the FP in Math1/L1 embryos. However, L1 mis-expression is unlikely to directly regulate the fasciculation state, per se, of these axons.
Previous studies have shown that the presumably long-range actions of Rig-1 are required for most spinal commissural axons to cross the floor plate in mouse embryos (Sabatier et al., 2004). In addition, contact dependent interactions between Axonin-1 and NrCAM are thought to facilitate midline crossing of commissural axons in the chick spinal cord (Stoeckli and Landmesser, 1995, 1997). Despite the incidence of stalling/inappropriate turning observed at the ipsilateral margin of the FP, and the rare occurrence of projection errors at the contralateral margin of the FP, most commissural axons ultimately crossed through the FP and followed the appropriate contralateral trajectories in Math1/L1 embryos. Therefore, it appears that the mis-expression of L1 does not significantly interfere with, and cannot overcome, the positive actions of Rig-1 and, possibly, TAG-1 (mouse axonin-1)-NrCAM interactions that most likely and ultimately promote midline crossing of commissural axons in Math1/L1 mouse embryos.
We initially carried out unilateral DiI labeling in open book preparations derived from Math1/L1 mouse embryos to determine the extent to which resident commissural axons exhibited pathfinding errors in the immediate vicinity of the ventral midline and/or on the contralateral side of the FP. This approach revealed that, in contrast to the normal projection patterns observed in wild-type mice, many commissural axons stalled or mis-projected into the longitudinal plane at the ipsilateral margin of the FP in Math1/L1 embryos. Since a given application of DiI likely labels a mixture of L1-mis-expressing and wild-type commissural axons, we also examined the pathfinding of GFP-labeled axons, and assessed the degree of overlap between DiI- and GFP-labeled axons, in open-book preparations derived from Math1/L1 embryos within a Math1/tauGFP background. This strategy was aimed at distinguishing among the following possibilities: 1) the axons that exhibit guidance errors at the ipsilateral margin of the FP mis-express L1; 2) mis-expression of L1 on neurons/axons derived from Math1 progenitors secondarily perturbs the pathfinding of other populations of commissural axons (likely follower axons) that do not mis-express L1 or 3) both L1-mis-expressing and wild type, L1-negative axons commit guidance errors in Math1/L1 mouse embryos. Our findings suggest that both L1 mis-expressing/GFP-labeled and L1-negative/GFP-negative axons stall or inappropriately turn into the longitudinal plane at the ipsilateral margin of the FP.
L1 is thought to regulate axon outgrowth and fasciculation through both homophilic and heterophilic interactions (Grumet and Sakurai, 1996; Hortsch, 1996; Itoh et al., 2004; Maness and Schachner, 2007). On either side of the ventral midline, commissural axons encounter other axons and lateral FP cells that express L1 and/or L1-binding proteins. Under normal circumstances, these axons and cells would be ignored by L1-negative commissural axons, which emanate from dorsal commissural neurons, as they extend into and across the midline. However, newly decussated commissural axons that upregulate L1 at their surfaces would engage, through either homophilic or heterophilic interactions, axons and cells at the contralateral FP boundary. In principle, these interactions could initiate a growth transition into the longitudinal axis (see Shirasaki and Murakami, 2001). Mis-expression of L1 on pre-crossing segments of commissural axons in Math1/L1 mice may permit these interactions to occur prematurely on the ipsilateral margin of the FP and consequently delay or otherwise disrupt the projection of axons directly through the midline. Importantly, axonal guidance defects were not observed within the CNS of a knock-in mouse line in which L1 is incapable of participating in homophilic adhesion (Itoh et al., 2004). Thus, premature L1-mediated heterophilic, but not homophilic, interactions are likely to account for the primary guidance defects observed in Math1/L1 mice. The L1-negative axons that also stall or inappropriately project into the longitudinal plane at the ipsilateral margin of the FP may represent follower axons, which normally engage in L1-independent interactions with pre-crossing segments of L1-negative pioneer axons in order to properly traverse the FP in wild-type embryos. Accordingly, in Math1/L1 embryos, the follower axons are mis-routed at the ipsilateral margin of the FP because the pioneer, L1 mis-expressing axons that they normally encounter have committed pathfinding errors in the immediate vicinity of the VM. We use novel in vitro assay systems, chick in ovo electroporation and a large array of mutant mice to identify guidance cues, and their corresponding receptors, which regulate the pathfinding of spinal commissural and spinal accessory motor axons. We are also investigating how dynamic changes in guidance receptor expression influence various aspects of commissural axon pathfinding in the spinal cord. In addition, we are identifying synaptic targets for genetically distinct commisural axons and elucidating the molecular logic that guides commissural axons from the spinal cord to the brain.
The polarized distribution of L1 in the embryonic mammalian spinal cord represents one of the earliest reported examples of an axon segment-specific expression pattern of a cell surface receptor in the developing CNS. The more recently described spatial segregation of the repulsive guidance receptors, Robo1, Robo2 and EphB, to post-crossing segments of commissural axons is likely to account, at least in part, for the observed altered responsiveness of these axons to midline-associated repellents (see Brittis et al., 2002; Dickson, 2002). By using Math1-Cre mice in conjunction with a variety of mice harboring floxed alleles of these guidance receptors, it should now be possible to selectively disrupt the corresponding commissural axon segment-specific expression patterns by controlling gene inactivation in a cell type-specific and temporally precise manner. Thus, a powerful feature of this experimental system is the ability to assess the consequences of eliminating gene function specifically in commissural neurons/axons, without interference from potential secondary effects associated with the complete absence of a gene (see Charron et al., 2003; Matsumoto et al., 2007).
In ongoing studies, we have adapted the gain-of-function approach introduced here to assess the role of other commissural axon-associated receptors in midline guidance. Specifically, we mis-expressed truncated, cytoplasmic domain-lacking forms of Robo proteins, which presumably function as dominant negative versions of these receptors, on commissural axons via unilateral electroporation in the embryonic chick spinal cord. Consistent with a critical and selective role for Robo signaling in axon guidance on the contralateral side of the FP, forced expression of dominant negative Robo proteins prevents post-crossing segments of commissural axons from extending away from the FP (S Reeber and Z Kaprielian, unpublished observations). These new observations further suggest that the targeted expression of guidance receptors to subsets of commissural neurons/axons in vivo represents a tractable strategy for examining the function(s) of a variety of axon navigation systems and their potential crosstalk during commissural axon pathfinding.
Materials and Methods
Generation of Math1/tauGFP and Math1/L1 transgenic mice
To create the Math1-tauGFP construct, 1,385 bp of sequence located ~3 kb 3’ of the Math1 coding sequence (nt 1-1385 from AF218258; Lumpkin et al., 2003) was placed 5’ of the BgtauGFP reporter construct. The BgtauGFP construct contains the β-globin basal promoter (from BGZA; Yee and Rigby, 1993), the Nco1/Xho1 sequence from Clontech (BD Biosciences; Palo Alto, CA), pIRES-EGFP, which contains EGFP with bovine growth hormone 3’ sequences, and a 1.2 kb bovine tau encoding sequence (Butner and Kirschner, 1991) at the N-terminus. The Math1-L1 construct was produced by fusing the L1 coding sequence (Moos et al., 1988) in-frame with Math1 cis-acting regulatory regions contained within the pATOLacZ8.5 plasmid. In pATOLacz8.5, the LacZ coding region is located between 2.6 kb of Math1 5’ flanking sequence and 5.7 kb of Math1 3’ flanking sequence that contains the Math1 enhancer (see Fig. 1D; Helms and Johnson, 1998; Helms et al., 2000). Transgene fragments were isolated from vector sequences and transgenic mice were generated using B6D2F1 oocytes according to previously described methods (see Verma-Kurvari et al., 1996). After a large number of injections, only one line expressing and transmitting the Math1/L1 transgene was obtained, presumably because expression of this particular transgene at high levels results in lethality.
In situ hybridization
The polymerase chain reaction was used to generate an approximately 1 kb cDNA fragment encoding a portion of mouse L1 (see Moos et al., 1988) and a digoxigenin-labeled riboprobe was then produced and in situ hybridization performed, all as previously described (Imondi et al., 2000).
Immunohistochemistry
Cryosections were generated and immunolabeled with the following primary antibodies: rat mAb anti-L1 (IgG; Chemicon, Temecula, CA), mouse mAb 4D7 [anti-TAG-1; IgM; Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA], mouse mAb AF-5 (anti-DCC; IgG; Oncogene, San Diego, CA), mouse mAb GAD-6 (anti-GAD-65; IgG; DSHB), mouse mAb 2H3 (anti-Neurofilament; IgG; DSHB), and mouse mAb EfB1-3 (anti-EphB; recognizes EphB1, EphB2 and EphB3; IgG; Jevince et al., 2006), as well as the appropriate goat anti-mouse or goat anti-rat, Cy3-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA), as previously described (Schubert and Kaprielian, 2001).
Anterograde DiI labeling
Commissural axons were anterogradely labeled through the iontophoretic application of DiI (1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine; Molecular Probes, Eugene, OR) into dorsal regions of fixed open-book spinal cord preparations as previously described (Imondi and Kaprielian, 2001).
Supplementary Material
(A-C) Transverse cryosections derived from wild-type mice that were labeled with an L1 riboprobe. (A) At E10, before most commissural axons have crossed the midline, L1 mRNA is predominantly expressed on ventral motor neurons (mn). L1 expression is also apparent in dorsal root ganglia (drg) at this developmental stage. During the period when commissural axons are actively crossing through the FP (between E12 and E13) L1 mRNA is expressed in a broad ventral domain that extends dorsolaterally into a region of the spinal cord occupied by multiple classes of contra- and ipsi-laterally projecting interneurons. Scale bar in (C), 100 μm (A-C).
Acknowledgments
We gratefully acknowledge Karen Jensen and the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, Iowa, 52242), for monoclonal antibodies 4D7, GAD-6, and 2H3. We also thank John Thomas for critical comments on the manuscript. This work was aided by grants from the New York State Department of Health Spinal Cord Injury Board (#C016886 and C018615) and the NIH (R01 NS038505) to Z.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bovolenta P, Dodd J. Guidance of commissural growth cones at the floor plate in embryonic rat spinal cord. Development. 1990;109:435–447. doi: 10.1242/dev.109.2.435. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- Brummendorf T, Kenwrick S, Rathjen FG. Neural cell recognition molecule L1: from cell biology to human hereditary brain malformations. Curr Opin Neurobiol. 1998;8:87–97. doi: 10.1016/s0959-4388(98)80012-3. [DOI] [PubMed] [Google Scholar]
- Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991;5:717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary T, Anderson KV. Patterning cell types in the dorsal spinal cord: what the mouse mutants say. Nat Rev Neurosci. 2003;4:289–297. doi: 10.1038/nrn1073. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen Sonic Hedgehog is an axonal chemoattractant that collaborates with Netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by Slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Grumet M, Sakurai T. Heterophilic interactions of the neural cell adhesion molecules Ng-CAM and Nr-CAM with neural receptors and extracellular matrix proteins. Semin Neurosci. 1996;8:379–389. [Google Scholar]
- Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13:14–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Hortsch M. The L1 family of neural cell adhesion molecules: old proteins performing new tricks. Neuron. 1996;17:587–593. doi: 10.1016/s0896-6273(00)80192-0. [DOI] [PubMed] [Google Scholar]
- Imondi R, Kaprielian Z. Commissural axon pathfinding on the contralateral side of the floor plate: a role for B-class ephrins in specifying the dorsoventral position of longitudinally-projecting commissural axons. Development. 2001;128:4859–4871. doi: 10.1242/dev.128.23.4859. [DOI] [PubMed] [Google Scholar]
- Imondi R, Wideman C, Kaprielian Z. Complementary expression of transmembrane ephrins and their receptors in the mouse spinal cord: a possible role in constraining the orientation of longitudinally projecting axons. Development. 2000;127:1397–1410. doi: 10.1242/dev.127.7.1397. [DOI] [PubMed] [Google Scholar]
- Itoh K, Cheng L, Kamei Y, Fushiki S, Kamiguchi H, Gutwein P, Stoeck A, Arnold B, Altevogt P, Lemmon V. Brain development in mice lacking L1-L1 homophilic adhesion. J Cell Biol. 2004;165:145–154. doi: 10.1083/jcb.200312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevince AR, Kadison SR, Pittman AJ, Chien C-B, Kaprielian Z. Distribution of EphB receptors and ephrin-B1 in the developing vertebrate spinal cord. J Comp Neurol. 2006;497:734–750. doi: 10.1002/cne.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadison SR, Kaprielian Z. Diversity of contralateral commissural projections in the embryonic rodent spinal cord. J Comp Neurol. 2004;472:411–422. doi: 10.1002/cne.20086. [DOI] [PubMed] [Google Scholar]
- Kadison SR, Murakami F, Matise MP, Kaprielian Z. The role of floor plate contact in the elaboration of contralateral commissural projections within the embryonic mouse spinal cord. Dev Biol. 2006;296:499–513. doi: 10.1016/j.ydbio.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Kaprielian Z, Runko E, Imondi R. Axon guidance at the midline choice point. Dev Dyn. 2001;221:154–181. doi: 10.1002/dvdy.1143. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS-Y, Culotti JG, Tessier-Lavigne M. Deleted in colorectal carcinoma (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expression Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Lustig M, Erskine L, Mason CA, Grumet M, Sakurai T. Nr-CAM expression in the developing mouse nervous system: ventral midline structures, specific fiber tracts, and neuropilar regions. J Comp Neurol. 2001;434:13–28. doi: 10.1002/cne.1161. [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Irie F, Inatani M, Tessier-Lavigne M, Yamaguchi Y. Netrin-1/DCC signaling in commissural axon guidance requires cell-autonomous expression of heparan sulfate. J Neurosci. 2007;27:4342–4350. doi: 10.1523/JNEUROSCI.0700-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M, Tacke R, Scherer H, Teplow D, Fruh K, Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson B. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Ma L, Brose K, Tamada A, Murakami F, Lee EY-HP, Tessier-Lavigne M. The divergent robo family protein Rig-1/Robo3 is a negative regulator of Slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Schubert W, Kaprielian Z. Identification and characterization of a cell surface marker for embryonic rat spinal accessory motor neurons. J Comp Neurol. 2001;439:368–383. doi: 10.1002/cne.1356. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Katsumata R, Murakami F. Change in chemoattractant responsiveness of developing axons at an intermediate target. Science. 1998;279:105–107. doi: 10.1126/science.279.5347.105. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Murakami F. Crossing the floor plate triggers sharp turning of commissural axons. Dev Biol. 2001;236:99–108. doi: 10.1006/dbio.2001.0321. [DOI] [PubMed] [Google Scholar]
- Silos-Santiago I, Snider WD. Development of interneurons with ipsilateral projections in embryonic rat spinal cord. J Comp Neurol. 1994;342:221–231. doi: 10.1002/cne.903420206. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by Slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14:1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Sonderegger P, Pollerberg GE, Landmesser LT. Interference with axonin-1 and NrCAM interactions unmasks a floor-plate inhibitory activity for commissural axons. Neuron. 1997;18:209–221. doi: 10.1016/s0896-6273(00)80262-7. [DOI] [PubMed] [Google Scholar]
- Tran TS, Phelps PE. Axons crossing in the ventral commissure express L1 and GAD65 in the developing rat spinal cord. Dev Neurosci. 2000;22:228–236. doi: 10.1159/000017445. [DOI] [PubMed] [Google Scholar]
- Verma-Kurvari S, Savage T, Gowan K, Johnson JE. Lineage-specific regulation of the neural differentiation gene MASH1. Dev Biol. 1996;180:605–617. doi: 10.1006/dbio.1996.0332. [DOI] [PubMed] [Google Scholar]
- Williams SE, Mason CA, Herrera E. The optic chiasm as a midline choice point. Curr Opin Neurobiol. 2004;14:51–60. doi: 10.1016/j.conb.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Yee SP, Rigby PW. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993;7:1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A-C) Transverse cryosections derived from wild-type mice that were labeled with an L1 riboprobe. (A) At E10, before most commissural axons have crossed the midline, L1 mRNA is predominantly expressed on ventral motor neurons (mn). L1 expression is also apparent in dorsal root ganglia (drg) at this developmental stage. During the period when commissural axons are actively crossing through the FP (between E12 and E13) L1 mRNA is expressed in a broad ventral domain that extends dorsolaterally into a region of the spinal cord occupied by multiple classes of contra- and ipsi-laterally projecting interneurons. Scale bar in (C), 100 μm (A-C).