Abstract
Congenital cardiac defects associated with increased pulmonary blood flow (Qp) produce pulmonary hypertension. We have previously reported attenuated endothelium-dependent relaxations in pulmonary arteries (PA) isolated from lambs with increased Qp and pulmonary hypertension. To better characterize the vascular alterations in the nitric oxide-superoxide system, 12 fetal lambs underwent in utero placement of an aortopulmonary vascular graft (shunt). Twin lambs served as controls. PA were isolated from these lambs at 4–6 wk of age. Electron paramagnetic resonance spectroscopy on fourth-generation PA showed significantly increased superoxide anion generation in shunt PA that were decreased to control levels following inhibition of nitric oxide synthase (NOS) with 2-ethyl-2-thiopseudourea. Pre-constricted fifth-generation PA rings were relaxed with a NOS agonist (A-23187), a nitric oxide donor [S-nitrosyl amino penicillamine (SNAP)], polyethylene glycol-conjugated superoxide dismutase (PEG-SOD), or H2O2. A-23187-, PEG-SOD-, and H2O2-mediated relaxations were impaired in shunt PA compared with controls. Pretreatment with PEG-SOD significantly enhanced the relaxation response to A-23187 and SNAP in shunt but not control PA. Inhibition of NOS with nitro-L-arginine or scavenging superoxide anions with tiron enhanced relaxation to SNAP and inhibited relaxation to PEG-SOD in shunt PA. Pretreatment with catalase inhibited relaxation of shunt PA to A-23187, SOD, and H2O2. We conclude that NOS catalyzes the production of superoxide anions in shunt PA. PEG-SOD relaxes shunt PA by converting these anions to H2O2, a pulmonary vasodilator. The redox environment, influenced by the balance between production and scavenging of ROS, may have important consequences on pulmonary vascular reactivity in the setting of increased Qp.
Keywords: congenital heart disease, hydrogen peroxide, nitric oxide
Altered pulmonary vascular reactivity and pulmonary hypertension are significant causes of morbidity and mortality in children with congenital cardiac defects that cause increased pulmonary blood flow. Pulmonary vascular endothelial dysfunction has been implicated in this pathophysiology. For example, children with increased pulmonary blood flow have early, selective impairment of endothelium-dependent pulmonary vasodilation, and adults with advanced pulmonary vascular disease have decreased gene expression of nitric oxide synthase (NOS) in the pulmonary vasculature (6, 13). More recent investigations have demonstrated a role for oxidative stress in the endothelial dysfunction associated with various vascular disorders, and thus reactive oxygen species (ROS) scavenging is a potential therapeutic strategy. However, the role of oxidative stress in the pulmonary vascular abnormalities associated with increased pulmonary blood flow, and the physiological effects of scavenging ROS in this setting, are unresolved.
Previously (25), we established a model of a congenital cardiac defect with increased pulmonary blood flow, using the placement of a large aortopulmonary vascular graft in the late-gestation fetal lamb. In the postnatal period, these lambs (shunt) display morphological and physiological features that mimic the human disease. With this model, we have demonstrated a selective impairment of endothelium-dependent pulmonary vasodilation in both intact shunt lambs and isolated pulmonary arteries (PA) harvested from shunt lambs, suggestive of decreased agonist-induced nitric oxide (NO) activity (3, 26, 31). However, shunt lambs also have increased endothelial NOS (eNOS) protein and mRNA levels (3). An uncoupling of eNOS, which can occur under conditions of decreased precursor and cofactor availability, resulting in the preferential production of superoxide anion over NO, may explain this paradigm of impaired endothelium-dependent vasodilation but increased eNOS gene expression (19, 21). In fact, we have previously demonstrated that superoxide dismutase (SOD), which catalyzes the dismutation of superoxide to hydrogen peroxide (H2O2), normalized endothelium-dependent relaxation in the PA of shunt lambs, implicating superoxide anion in the altered reactivity (31). In addition, with the use of whole lung biopsies, we demonstrated that NOS is a source of superoxide production in shunt lambs. However, the role of NOS in the production of ROS in the pulmonary vasculature, as well as the role of ROS in altered pulmonary vascular reactivity under conditions of increased pulmonary blood flow, remain to be clarified.
Thus the objectives of the current study were 1) to evaluate superoxide anion production and the role of NOS in its generation in shunt-isolated PA; 2) to confirm the effect of SOD supplementation and NOS inhibition on the relaxation to eNOS agonists and NO donors in PA isolated from shunt and control lambs; and 3) mainly to determine the relaxation effects of a membrane-permeable form of SOD in both control and shunt PA and its mechanism of action.
METHODS
The following protocols and procedures were approved by the Institutional Animal Care Committee at the State University of New York at Buffalo. Twelve mixed-breed time-dated pregnant ewes were obtained from Swartz Family Farm (Attica, NY). At ~139 days gestation (term ~145 days), an 8-mm Gore-Tex vascular graft was placed between the fetal aorta and pulmonary artery as previously described (25). The fetus was placed back in the uterus. Four to six weeks after spontaneous delivery (mean age, 5.4 ± 0.24 wk), the shunt lambs were killed by rapid exsanguination under pentobarbital anesthesia. Thirteen control lambs (usually twin/triplet controls) were also studied for comparison.
In vivo electron paramagnetic resonance measurement of super-oxide anions
Given the millisecond-range half-life of superoxide in situ, electron paramagnetic resonance (EPR) assay involves the approach of using a comparatively specific superoxide spin-trap, 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl pyrrolidine HCl (CMH hydrochloride; Axxora) (8, 9, 11, 16, 18), to generate a stable chemical product by a general method that we have used previously with in vitro cell cultures (35, 36). Approximately 0.2 g of tissue was sectioned from fresh-frozen samples of fourth-generation PA and was immediately immersed in either normal EPR buffer [PBS supplemented with 5 μM diethydithiocarbamate (Sigma-Aldrich) and 25 μM desferrioxamine (Sigma-Aldrich)]. To determine both specificity and origin of superoxide, additional sample groups from both control and shunt lambs were immersed in either EPR buffer supplemented with either 100 U/ml membrane-permeable form of SOD [polyethylene glycol-conjugated SOD (PEG-SOD)] to determine the superoxide specificity of CMH or 100 μM 2-ethyl-2-thiopseudourea (ETU), a specific inhibitor of NOSs (12, 28), to ascertain the relative contribution of uncoupled NOSs to overall superoxide production. All samples were incubated for 30 min on ice, then homogenized for 30 s with a VWR PowerMAX AHS 200 tissue homogenizer. Following incubation, samples were analyzed for protein content by using Bradford analysis (Bio-Rad). Sample volumes were then adjusted with EPR buffer plus 25 mg/ml CMH hydrochloride to achieve equal protein content and a final CMH concentration of 5 mg/ml. Samples were further incubated for 60 min on ice and then were centrifuged at 14,000 g for 15 min at room temperature. Supernatant from each sample (35 μl) was loaded into a 50-μl capillary tube and was analyzed with a MiniScope MS200 ESR (Magnettech, Berlin, Germany) at a microwave power of 40 mW, modulation amplitude of 3,000 mG, and modulation frequency of 100 kHz, with a magnetic strength of 333.95–339.94 mT. Resulting EPR spectra were analyzed by using ANALYSIS v.2.02 software (Magnettech), whereby the EPR maximum and minimum spectral amplitudes for the 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl pyrrolidine (CM) · superoxide spin-trap product waveform were quantified. Experimental groups were then compared for differences in amplitude by using statistical analysis.
Isolated PA studies
Fifth-generation PA (inner diameters of ~1 mm) were dissected, isolated, and cut into rings as described previously (30). Rings were suspended in water-jacketed chambers filled with aerated (94% O2-6% CO2), modified Krebs-Ringer solution (in mM: 118 sodium chloride, 4.7 potassium chloride, 2.5 calcium chloride, 1.2 magnesium sulfate, 1.2 potassium biphosphate, 25.5 sodium bicarbonate, and 5.6 glucose). A continuous recording of isometric force generation was obtained by tying each vessel ring to a force-displacement transducer (model UC2; Statham Instruments, Hato Rey, PR) that was connected to a recorder (Gould Instrument Systems, Valley View, OH). After the arterial rings were mounted, they were allowed to equilibrate for 20 min in the bathing solution. A micrometer was used to stretch the tissues repeatedly in small increments over the following 45 min until resting tone remained stable at a passive tension of 0.8 g. Preliminary experiments determined that this procedure provided optimal length for generation of active tone to exogenous norepinephrine.
The following pharmacological agents were used: indomethacin, dl-propranolol, norepinephrine hydrochloride (NE), calcium ionophore A-23187, and NO donor S-nitrosyl amino penicillamine (SNAP), PEG-SOD, PEG-catalase, H2O2, tiron, and the NOS antagonist, Nω-nitro-L-arginine (L-NNA). All drugs were obtained from Sigma-Aldrich. SNAP was dissolved first in a small quantity of DMSO and then diluted in distilled water. Indomethacin was dissolved in ethanol, and L-NNA was dissolved in warmed Krebs solution. All other drugs were dissolved in distilled water. Ethanol and DMSO, at the concentrations used in these experiments, did not alter the preexisting tone of the PA.
Isolated PA were pretreated with indomethacin (10−5 M) to prevent the formation of vasoactive prostaglandins and propranolol (10−6 M) to block β-adrenergic receptors. The arteries were first constricted with increasing doses of NE (10−8–10−6 M) and were relaxed with increasing concentrations of either A-23187 (10−8–10−6 M), SNAP (10−8–10−5.5 M), PEG-SOD (0.75 U/ml to 75 U/ml), or H2O2 (10−6–10−4 M). According to protocol, some vessels were pretreated with L-NNA (10−3 M, to block NOS), tiron (10−5 M, to scavenge superoxide anions), PEG-catalase (1,200 U/ml), or PEG-SOD (37.5 U/ml). These experiments were carried out in a darkened room due to the light sensitivity of L-NNA.
Statistical analysis
All data are expressed as means ± SE, with n representing the number of animals studied. Statistical comparisons of the curves were performed with repeated-measures ANOVA as appropriate. Statistical analysis was performed with StatView software (Abacus Concepts, Berkley, CA). Significance was accepted at P < 0.05.
RESULTS
Superoxide anion production is higher in shunt PA compared with control PA
The waveform amplitude by EPR inhibitable by PEG-SOD was significantly higher in shunt PA compared with control PA (Fig. 1A). In shunt PA, pretreatment with PEG-SOD decreased the amplitude to control levels, suggesting specific quenching of superoxide anions by PEG-SOD. Pretreatment of control PA with PEG-SOD had no effect.
Fig. 1.
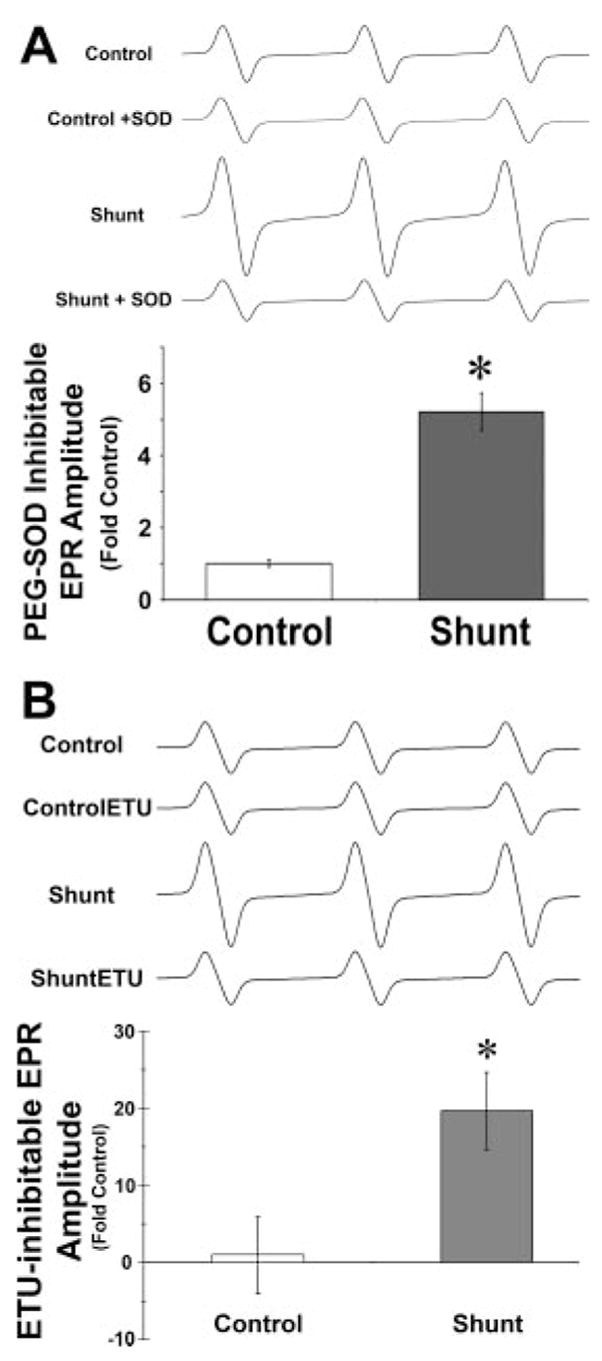
Electron paramagnetic resonance (EPR) spectroscopy measurement of superoxide anions in pulmonary arteries (PA) isolated from control lambs (control) and lambs with fetal placement of an aortopulmonary shunt (shunt) and the effect of pretreatment with polyethylene glycol-conjugated superoxide dismutase (PEG-SOD) (A) and NOS inhibitor 2-ethyl-2-thiopseudourea (ETU) (B). The decrease in waveform amplitude in control PA following pretreatment with PEG-SOD or ETU is normalized to 1.0. Data are presented as mean change in the amplitude of the EPR waveform of CM · product ± SD, following inhibition with PEG-SOD or ETU, from n = 6 control and n = 6 shunt lambs. Representative waveforms from each group are included. *P < 0.05 compared with control.
Superoxide anion production in shunt PA is decreased by NOS inhibition
Pretreatment with NOS inhibitor ETU significantly reduced the amplitude in shunt PA (Fig. 1B). This indicates that NOS is a significant source of superoxide anions in shunt PA. Pretreatment of control PA with ETU had no effect. The ETU-inhibitable component of this waveform amplitude is significantly higher in shunt PA compared with controls.
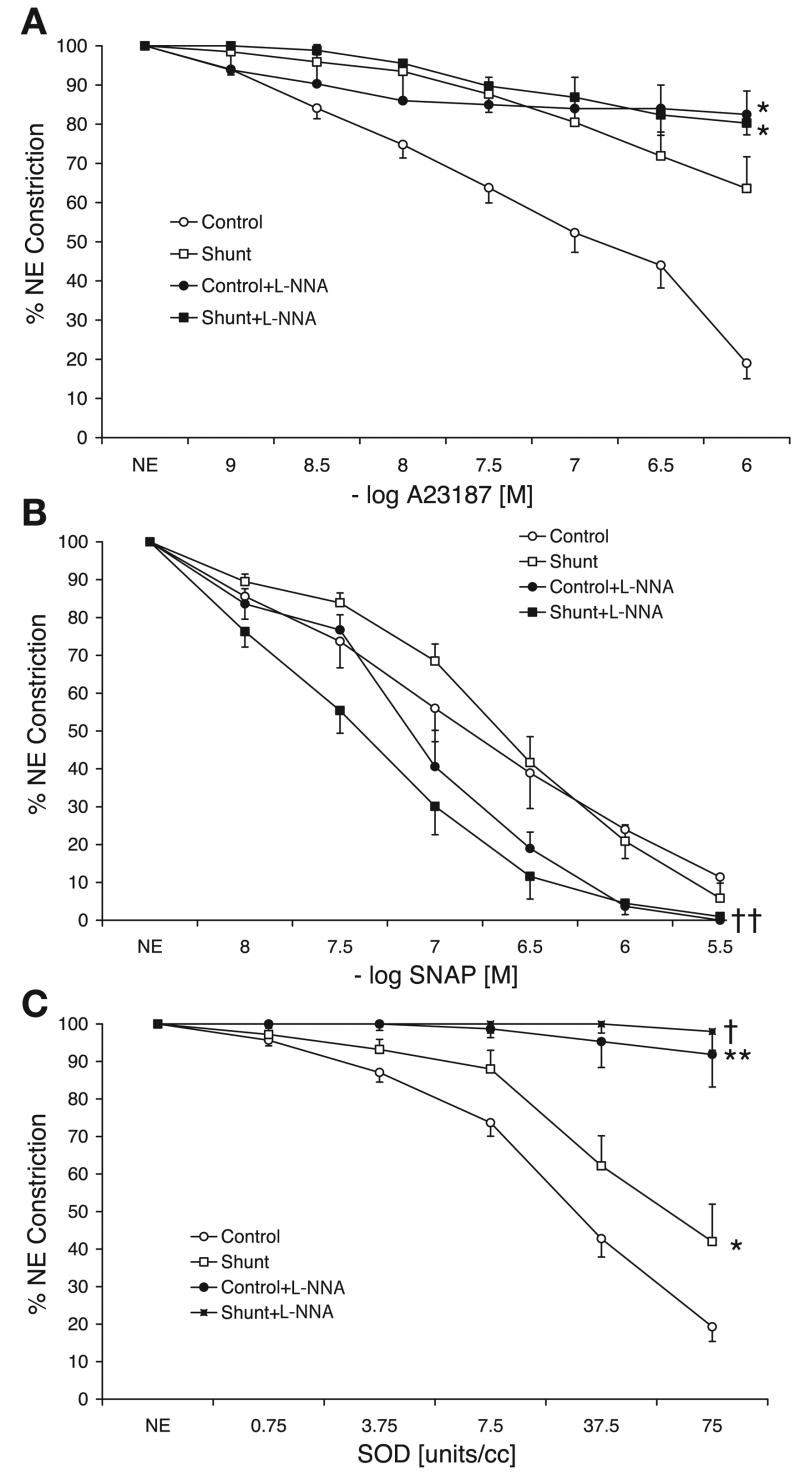
SOD enhances NO-mediated relaxation in shunt PA
We have previously reported that the relaxation response to NOS stimulant A-23187 is impaired in shunt PA (2, 31). Pretreatment with PEG-SOD significantly enhanced the relaxation response in shunt but not control PA (Fig. 2A). Similarly, NO donor SNAP relaxation is significantly enhanced by pretreatment with PEG-SOD in shunt PA (Fig. 2B). Pretreatment with PEG-SOD also enhanced the relaxation response to SNAP in control PA, but this effect failed to reach significance (P = 0.06).
Fig. 2.
Concentration-response curves to endothelial nitric oxide synthase stimulant and calcium ionophore A-23187 (A) and nitric oxide donor S-nitrosyl amino penicillamine (SNAP; B) in PA isolated from 4- to 6-wk-old control lambs an shunt lambs. All PA were pretreated with indomethacin (10−5 M) and propranolol (10−6 M) and were contracted with norepinephrine (NE; 3 × 10−7 M). Some PA were pretreated with 37.5 units/ml of PEG-SOD. There was a tendency toward enhanced relaxations in control PA following pretreatment with PEG-SOD (P = 0.06). *P < 0.05 compared with control and †P < 0.05 compared with shunt. Data were obtained from 13 control and 12 shunt lambs.
Superoxide anion scavenger tiron enhances exogenous NO-but not NOS-mediated relaxation in shunt PA
Pretreatment of shunt PA with superoxide scavenger tiron significantly enhanced the relaxation response to SNAP. Similar to PEG-SOD, tiron did not enhance SNAP-mediated relaxation in control PA (Fig. 3B). In sharp contrast, pretreatment with tiron did not significantly alter the impaired A-23187-mediated relaxation in shunt PA and decreased A-23187-mediated relaxation in control PA (Fig. 3A). PEG-SOD relaxed control PA significantly better than shunt PA, and both these relaxations were significantly inhibited by pretreatment with tiron (Fig. 3C).
Fig. 3.
Concentration-response curves to A-23187 (A), SNAP (B), and PEG-SOD (C) in control and shunt PA with or without pretreatment with superoxide scavenger tiron (10−5 M). Data were obtained from 13 control lambs and 8 shunt lambs. *P < 0.05 and **P < 0.01 compared with control, and †P < 0.05 compared with shunt PA.
NOS inhibition enhances NO-mediated relaxation in shunt PA
A nonspecific NOS inhibitor, L-NNA, as expected, blocked relaxation to A-23187 in both shunt and control PA. However, L-NNA enhanced the relaxation to SNAP in shunt but not control PA (Fig. 4B), similar to that observed following pre-treatment with SOD and tiron. Relaxation of both control and shunt PA to PEG-SOD were significantly inhibited by pretreatment with L-NNA (Fig. 4C).
Fig. 4.
Concentration-response curves to A-23187 (A), SNAP (B), and PEG-SOD (C) in control and shunt PA with or without pretreatment with nitric oxide synthase inhibitor nitro-L-arginine (L-NNA; 10−3 M). Data were obtained from 13 control lambs and 10 shunt lambs. *P < 0.05 and **P < 0.01 compared with control, and †P < 0.05 and ††P < 0.001 compared with shunt PA.
Pretreatment with catalase inhibits relaxation to A-23187
Pretreatment with PEG-catalase significantly inhibited the relaxation to A-23187 and PEG-SOD in both control and shunt PA and almost completely blocked relaxation to these agents in shunt PA (Fig. 5, A and C). SNAP-mediated relaxation was not altered by PEG-catalase (Fig. 5B). H2O2 relaxed control PA significantly better than shunt PA, and both these relaxations were completely blocked by pretreatment with PEG-catalase (Fig. 5D).
Fig. 5.
Concentration-response curves to A-23187 (A), SNAP (B), PEG-SOD (C), and hydrogen peroxide (D) in control and shunt PA with or without pretreatment with PEG-catalase (1,200 units/ml). *P < 0.05 and **P < 0.005 compared with control, and †P < 0.05 compared with shunt PA.
DISCUSSION
Together, these data indicate that NOS-generated superoxide and subsequent H2O2 production are integral to the mechanisms mediating PA function under conditions of increased pulmonary blood flow. We found that superoxide levels are increased in shunt PA and that NOS is a source of superoxide production. This suggests that NOS is uncoupled, such that with stimulation it produces superoxide in preference to NO. Thus diminished NO production by NOS likely contributes in part to attenuated agonist-induced endothelium-dependent relaxation in PA. However, the role of superoxide appears to be more complicated. Scavenging superoxide in a manner that does not lead to H2O2 production does not attenuate impaired endothelium-dependent relaxation in PA, whereas the dismutation of superoxide to H2O2 does, suggesting that the latter effect is mediated by H2O2-induced relaxation. At the same time, superoxide scavenging, independent of H2O2, augments endothelium-independent relaxation to exogenous NO, suggesting that superoxide may decrease bioavailable NO and thus may also contribute to impaired shunt PA relaxation.
With the use of fluorescent dye techniques, we previously demonstrated that peripheral lung samples taken from shunt lambs have increased levels of superoxide anion (14). In addition, treatment with NOS antagonists before staining with superoxide-sensitive dyes decreased fluorescence, suggesting that NOS was a potential source of superoxide in shunt peripheral lung. However, this technique could not isolate the precise site of superoxide production within the lung (14). In the current study, we used EPR to demonstrate for the first time that superoxide anion generation is increased in the PA isolated from shunt lambs. In addition, since NOS antagonists normalize superoxide anion levels, our data suggest that the increased production in the vessels is dependent on endogenous NOS activity (Fig. 1). It should be noted that these determinations reflect basal amounts of superoxide anion generation, as opposed to generation that occurs on agonist stimulation. Further studies are warranted to measure superoxide generation with agonist stimulation.
Impairment of endothelium-dependent vasodilation is a hallmark of endothelial dysfunction. Endothelium-dependent vasodilators, such as acetylcholine and the calcium ionophore A-23187, induce vasodilation by the activation of NOS and the liberation of NO. Several years ago, Celemajer et al. (6) demonstrated an attenuated pulmonary vasodilating response to acetylcholine in infants and children with congenital cardiac defects, resulting in increased pulmonary blood flow. Subsequently, we found a similar impairment of endothelium-dependent vasodilation in both the intact shunt lamb and in isolated PA harvested from shunt lambs (2, 31). In the current study, we demonstrate that stimulation of NOS generates superoxide anions, suggesting that NOS is uncoupled (Fig. 1B). Previous studies have demonstrated that suboptimal concentrations of the precursor L-arginine and cofactors may result in uncoupling of NOS (17, 22). The exact etiology of NOS uncoupling in this setting is unclear, but we have previously demonstrated both decreased L-arginine levels and alterations in BH4 metabolism in shunt lambs (14, 26). Thus the impaired relaxation to A-23187 may result, in part, from decreased NO production from NOS. Interestingly, we found that the dismutation of superoxide to H2O2 by SOD pretreatment normalized A-23187-mediated relaxation in shunt PA and that scavenging superoxide anions by tiron or metabolizing H2O2 with catalase inhibited relaxation to A-23187 in shunt pulmonary arteries. This suggests that H2O2 plays a crucial role in SOD-mediated restoration of endothelium-dependent relaxation of shunt pulmonary arteries.
Previously, we have demonstrated that despite alterations in the downstream mediators of the NO-cGMP cascade, endothelium-independent vasodilation is intact in shunt lambs (2, 4, 31). For example, in the intact shunt lamb, inhaled NO dilates the pulmonary circulation similar to controls. Similarly, SNAP-induced dilation is similar in PA isolated from shunt and control lambs (2, 31). We recapitulate these data in Fig. 2B. Interestingly, the current study also demonstrates that endothelium-independent relaxations to NO donors (SNAP) are enhanced in shunt PA by pretreatment with SOD, tiron, and L-NNA. Because these agents all reduce the concentration of superoxide anions (10) either by converting them to H2O2 (SOD), scavenging them (tiron), or reducing their production through inhibition of NOS (L-NNA), these data suggest that under basal conditions, exogenous NO is partly inactivated in shunt lambs by superoxide anions. This is consistent with our previous data in a lamb model of persistent pulmonary hypertension of the newborn (following in utero constriction of the ductus arteriosus), in which the pulmonary vasodilating response to inhaled NO was augmented by intratracheal administration of SOD (20). In children with congenital heart disease and elevated pulmonary vascular resistance, the combination of oxygen and inhaled NO is thought to produce maximal pulmonary vasodilation and is therefore utilized for pre- and postoperative reactivity testing, as well as perioperative pulmonary vasodilator therapy (1, 34). The current data suggest that the addition of ROS scavengers may augment the pulmonary vasodilating response of inhaled NO and thereby optimize its utility in this patient population. Further in vivo studies are warranted.
The exogenous administration of SOD is a promising new therapy for pulmonary vascular disease. For example, preclinical data suggest a role in the treatment of persistent pulmonary hypertension of the newborn (20, 29), and administration in preterm neonates demonstrates its safety and potential benefit on pulmonary function (7). Vascular relaxation to SOD is traditionally thought to be secondary to sparing endogenous NO (15, 23). However, SOD converts superoxide anions to H2O2, a potential pulmonary vasodilator (22). Our current data suggest that in control PA, both the above-mentioned mechanisms, i.e., sparing of NO (not inhibited by catalase) and formation of H2O2 (inhibited by catalase), play an important role. However, in shunt PA, relaxation to PEG-SOD is completely blocked by catalase, suggesting that H2O2 is solely responsible for this relaxation (Fig. 5, C and D). Pretreatment with L-NNA completely blocks relaxation response to PEG-SOD in control and shunt PA (Fig. 4C). Thus NOS appears to be a significant source of NO and superoxide anions in both control and shunt lambs.
We have previously shown (14) that superoxide but not H2O2 levels are increased in 4-wk-old shunt lamb lungs without any change in SOD or catalase activity. However, administration of SOD converts these elevated superoxide anion levels to H2O2, a potential pulmonary vasodilator. In Fig. 5, we demonstrate that H2O2 induces dose-dependent relaxation in isolated PA from both control and shunt lambs. However, the effect is attenuated in shunt compared with control lambs. Previous studies in isolated rabbit lungs demonstrate that H2O2 induces endothelium-independent pulmonary vasodilation via the activation of soluble guanylate cyclase and the subsequent increase in cGMP concentrations (5). The etiology of the attenuated response in shunt lambs is unclear and warrants further study.
It should be noted that several vascular responses, including H2O2, are dependent on the oxygen environment. Many laboratories (including ours) that study isolated vessels in conventional tissue baths use a 94% oxygen and 6% CO2 gas mixture (27, 32, 33). The PO2 of the buffer solution bathing the tissue is close to 500 mmHg, and PCO2 is ~40 mmHg. This approach is based on the assumption that the inner core of smooth muscle cells in the vessel may be relatively hypoxic because of lack of perfusion through the vasa vasorum. We acknowledge the possibility that a high buffer PO2 might have affected our results. However, similar conditions were used to study PA isolated from control and shunt lambs. Also, many patients with pulmonary hypertension are treated with high concentrations of oxygen, resulting in high alveolar PO2 levels.
In summary, we demonstrate that eNOS-ROS interactions mediate the altered endothelium-dependent and -independent vascular relaxations noted in lambs with increased pulmonary blood flow. We have previously shown (14) that superoxide but not H2O2 levels are increased in 4-wk-old shunt lamb lungs without any change in SOD or catalase activity. These elevated superoxide levels are due to increased production from uncoupled NOS and other sources, which include NADPH oxidase. Conversion of these elevated superoxide anion levels to a pulmonary vasodilator such as H2O2 by exogenous SOD either administered by an intravascular (24) or intratracheal (20) route may normalize and/or augment pulmonary vasodilator responses. Therefore, coadministration of SOD should be considered a potential therapeutic option in the management of pulmonary hypertension with increased pulmonary blood flow associated with congenital heart disease, and it warrants further study. In addition, these data suggest that the redox environment, which is influenced by the balance between ROS production and scavenging, may have important consequences on pulmonary vascular reactivity in the setting of increased pulmonary blood flow.
Acknowledgments
GRANTS
This work was supported by Grants HL-54705 (to R. H. Steinhorn) HL60190 (to S. M. Black), and HL-61284 (to J. R. Fineman) from the National Heart, Lung, and Blood Institute and by a Transatlantic Network Development Grant from the LeDucq Foundation (to S. M. Black and J. R. Fineman).
References
- 1.Atz AM, Adatia I, Lock JE, Wessel DL. Combined effects of nitric oxide and oxygen during acute pulmonary vasodilator testing. J Am Coll Cardiol. 1999;33:813–819. doi: 10.1016/s0735-1097(98)00668-8. [DOI] [PubMed] [Google Scholar]
- 2.Black SM, Bekker JM, McMullan DM, Parry AJ, Ovadia B, Reinhartz O, Lakshminrushimha S, Mata-Greenwood E, Steinhorn RH, Fineman JR. Alterations in nitric oxide production in 8-wk-old lambs with increased pulmonary blood flow. Pediatr Res. 2002;52:233–244. doi: 10.1203/00006450-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Black SM, Fineman JR, Steinhorn RH, Bristow J, Soifer SJ. Increased endothelial NOS in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol. 1998;275:H1643–H1651. doi: 10.1152/ajpheart.1998.275.5.H1643. [DOI] [PubMed] [Google Scholar]
- 4.Black SM, Sanchez LS, Mata-Greenwood E, Bekker JM, Steinhorn RH, Fineman JR. sGC and PDE5 are elevated in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1051–L1057. doi: 10.1152/ajplung.2001.281.5.L1051. [DOI] [PubMed] [Google Scholar]
- 5.Burke-Wolin T, Abate CJ, Wolin MS, Gurtner GH. Hydrogen peroxide-induced pulmonary vasodilation: role of guanosine 3′,5′-cyclic mono-phosphate. Am J Physiol Lung Cell Mol Physiol. 1991;261:L393–L398. doi: 10.1152/ajplung.1991.261.6.L393. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Cullen S, Deanfield JE. Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation. 1993;87:440–446. doi: 10.1161/01.cir.87.2.440. [DOI] [PubMed] [Google Scholar]
- 7.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics. 2003;111:469–476. doi: 10.1542/peds.111.3.469. [DOI] [PubMed] [Google Scholar]
- 8.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 9.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 10.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 11.Fink B, Dikalov S, Bassenge E. A new approach for extracellular spin trapping of nitroglycerin-induced superoxide radicals both in vitro and in vivo. Free Radic Biol Med. 2000;28:121–128. doi: 10.1016/s0891-5849(99)00228-2. [DOI] [PubMed] [Google Scholar]
- 12.Garvey EP, Oplinger JA, Tanoury GJ, Sherman PA, Fowler M, Marshall S, Harmon MF, Paith JE, Furfine ES. Potent and selective inhibition of human nitric oxide synthases. Inhibition by non-amino acid isothioureas. J Biol Chem. 1994;269:26669–26676. [PubMed] [Google Scholar]
- 13.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 14.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 15.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 16.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2−from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 17.Konduri GG, Ou J, Shi Y, Pritchard KA., Jr Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2003;285:H204–H211. doi: 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- 18.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354. doi: 10.1016/j.bcp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 20.Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1370–1377. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mata-Greenwood E, Jenkins C, Farrow KN, Konduri GG, Russell JA, Lakshminrusimha S, Black SM, Steinhorn RH. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol. 2006;290:L232–L241. doi: 10.1152/ajplung.00393.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moncada S, Palmer RM, Gryglewski RJ. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1986;83:9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oishi P, Grobe A, Benavidez E, Ovadia B, Harmon C, Ross GA, Hendricks-Munoz K, Xu J, Black SM, Fineman JR. Inhaled nitric oxide induced NOS inhibition and rebound pulmonary hypertension: a role for superoxide and peroxynitrite in the intact lamb. Am J Physiol Lung Cell Mol Physiol. 2006;290:L359–L366. doi: 10.1152/ajplung.00019.2005. [DOI] [PubMed] [Google Scholar]
- 25.Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts. A model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation. 1995;92:606–613. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- 26.Reddy VM, Wong J, Liddicoat JR, Johengen M, Chang R, Fineman JR. Altered endothelium-dependent responses in lambs with pulmonary hypertension and increased pulmonary blood flow. Am J Physiol Heart Circ Physiol. 1996;271:H562–H570. doi: 10.1152/ajpheart.1996.271.2.H562. [DOI] [PubMed] [Google Scholar]
- 27.Schindler MB, Hislop AA, Haworth SG. Postnatal changes in response to norepinephrine in the normal and pulmonary hypertensive lung. Am J Respir Crit Care Med. 2004;170:641–646. doi: 10.1164/rccm.200311-1551OC. [DOI] [PubMed] [Google Scholar]
- 28.Southan GJ, Szabo C, Thiemermann C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br J Pharmacol. 1995;114:510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:834–839. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 30.Steinhorn RH, Morin FC, 3rd, Gugino SF, Giese EC, Russell JA. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol Heart Circ Physiol. 1993;264:H2162–H2167. doi: 10.1152/ajpheart.1993.264.6.H2162. [DOI] [PubMed] [Google Scholar]
- 31.Steinhorn RH, Russell JA, Lakshminrusimha S, Gugino SF, Black SM, Fineman JR. Altered endothelium-dependent relaxations in lambs with high pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2001;280:H311–H317. doi: 10.1152/ajpheart.2001.280.1.H311. [DOI] [PubMed] [Google Scholar]
- 32.Villamor E, Degraeuwe PL, De Mey JG, Blanco CE. Vascular reactivity of pulmonary arteries from premature lambs subjected to liquid ventilation. Biol Neonate. 2003;84:172–178. doi: 10.1159/000071953. [DOI] [PubMed] [Google Scholar]
- 33.Villamor E, Kessels CG, Fischer MA, Bast A, de Mey JG, Blanco CE. Role of superoxide anion on basal and stimulated nitric oxide activity in neonatal piglet pulmonary vessels. Pediatr Res. 2003;54:372–381. doi: 10.1203/01.PDR.0000077481.15081.C8. [DOI] [PubMed] [Google Scholar]
- 34.Wessel DL, Adatia I, Giglia TM, Thompson JE, Kulik TJ. Use of inhaled nitric oxide and acetylcholine in the evaluation of pulmonary hypertension and endothelial function after cardiopulmonary bypass. Circulation. 1993;88:2128–2138. doi: 10.1161/01.cir.88.5.2128. [DOI] [PubMed] [Google Scholar]
- 35.Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2007;292:L165–L177. doi: 10.1152/ajplung.00459.2005. [DOI] [PubMed] [Google Scholar]
- 36.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol. 2006;291:C555–C568. doi: 10.1152/ajpcell.00509.2005. [DOI] [PubMed] [Google Scholar]