Abstract
Although the adaptive value of flight may seem obvious, it is the most difficult behaviour of birds to monitor. Here, we describe a technique to quantify the frequency and the duration of flights over several months by implanting a data logger that records heart rate (fH), hydrostatic pressure (diving depth) and the body angle of a large sea duck species, the common eider (Somateria mollissima). According to the mean fH recorded during flight and the parameters recorded to identify the fH flight signature, we were able to identify all flights performed by 13 individuals during eight months. We cumulated local flight time (outside migrations) and found that activity occurs primarily during dawn and morning and that flying activities are strongly related to diving activities (Pearson's r=0.88, permutation test p<0.001). This relationship was interpreted as a consequence of living in a dynamic environment where sea currents move the ducks away from the food patches. We believe that the technique described here will open new avenues of investigation in the adaptive value of flight.
Keywords: common eider, data logger, diving behaviour, flying behaviour, free-living birds, heart rate
1. Introduction
The main functions of flight are to increase foraging success, reduce the probability of predation and facilitate long-distance movements to escape adverse conditions (Pomeroy 1990). However, to link flight to one of these advantages is difficult owing to technical and logistical difficulties. Radar and satellite telemetry have been used to analyse the orientation and duration of long migrating flights (Casement 1966; Butler et al. 1998; Hays et al. 2001), but none provided detailed information about flights of shorter duration. To quantify short local flights, researchers have used visual observations (Walsberg 1983) and telemetry (Ackerman et al. 2006), but these methods are probably biased for most species and ineffective to monitor marine birds.
For almost four decades, the fact that heart rate increases to high levels during forward flapping flight has been documented by physiologists studying both short (Lord et al. 1962; Kanwisher et al. 1978; Bevan et al. 1997) and migration flights (Butler et al. 1998). However, the heart rate (fH) signature of flight (shape of trace in relation to time) has never been quantified and used as a means of identifying and compiling all flights performed by a bird species.
We studied the flying and diving behaviour of common eider (Somateria mollissima), a large sea duck species that spend most of its time (11 months) at sea. We describe the characteristics of the flight signature and present data of observations during the post-hatching period in order to discriminate fH reached during flight from that recorded during other behaviours. We show the potential of this new technique by analysing the occurrence of flight behaviour in relation to time of the day and foraging behaviour. We tested the hypothesis that flight behaviour in this species is mostly used as a way to improve foraging opportunities.
2. Material and methods
In 2003 and 2004, 20 and 10 female eiders, respectively, were equipped with data loggers (DLs, 36×28×11 mm; 21 g, i.e. less than 1.2% of body mass, Woakes et al. 1995). The DLs recorded heart rate (0.5 Hz), pressure (dive depth, 0.5 Hz) and body angle (1 Hz, those used in 2004 only). The body angle switch turned on due to acceleration and speed or when the DL is at an angle of +45 or −45°. Using a before–after design with a control group, it was impossible to detect any negative impact on laying date, clutch size and hatching success for individuals carrying a DL for one full year (Guillemette et al. 2002). Furthermore, the DLs used in this study were implanted in the body cavity of the experimental bird, with no external protrusions, thus conserving aerodynamic and hydrodynamic properties of the study animals (Guillemette et al. 2002).
Only females implanted in 2003 were included in the analysis of flight time. Eighteen females returned to the colony (90%) for laying, 17 of which were recaptured 1 year later and their DLs were removed. Only 13 loggers with their memory full or almost full (190–220 days) were kept for flight analysis, data spanning from May 2003 until the middle of December 2003. We kept only local flights to test our hypothesis (99% of all flights) that flight behaviour was mostly linked to foraging behaviour and demand.
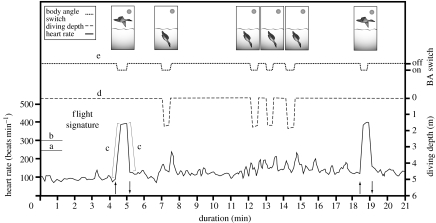
We compared fH recorded during flight to fH recorded during different activities by conducting focal observations (only one animal at a time) on 10 (2003) and 4 (2004) experimental females during the post-hatching period (total of 118 h of observation). Behaviours were assigned to one of the 11 categories described in the electronic supplementary material. By using the signature of recorded flight activity performed during the post-hatching period, we found the set of parameters (figure 1) giving the best description of the shape of trace in relation to time, and they were programmed in a purpose-designed software for the extraction of all flights performed by each female. The set of parameters was then validated by visual analysis of traces in all recording days in each female to ensure that the parameters selected only the fH signatures related to flight.
Figure 1.
Traces of heart rate (fH), diving depth and body angle from a female common eider during dives and forward flapping flight. The flight signature is determined by the following parameters: (a) fH threshold equal to or above 250 beats per minute, (b) fH plateau threshold equal to or above 300 beats per minute, (c) fH ascending and descending slopes equal to or above 10 beats per minute per second (absolute values), (d) standard deviation of diving depth up to 0.1 m (§2), and (e) body angle switch (BA switch) turned on. The upward and downward pointing arrows indicate the point of take-off and landing, respectively.
Owing to the extensive recording period, the duration of daylight varied along the sampling period. Thus, we had to adjust take-off time of each flight to determine the percentage of flights performed according to sunrise and sunset by using the equation: adjusted time=(t0−Hsunrise)/(Hsunset−Hsunrise), where t0 is the take-off time, Hsunrise sunrise time and Hsunset sunset time (dawn is between −0.2 and 0.0, 0.0 is sunrise, 0.5 is midday, 1.0 is sunset, dusk is between 1.0 and 1.2, and outside these boundaries it is night).
3. Results
Parameters found to identify fH flight signature during forward flapping flight were defined using focal observations and are summarized in figure 1. The almost instantaneous increase in fH during take-off and the large decrease during landing are quantified with increasing and decreasing slopes equal to or above 10 beats per minute per second (in absolute values). The take-off ascending slope exceeded 250 beats per minute and the fH plateau reached after take-off and recorded during forward flapping flight exceeded a threshold of 300 beats per minute. To eliminate some fH events related to diving bouts or bathing behaviour with head submersion that are very similar to flights (Stephenson et al. 1986), we removed all fH events with unstable hydrostatic pressure (standard deviation of diving depth equal to or above 0.1 m). The body angle switch turned on while birds were taking off from land, stayed on during the majority of plateau phase duration and turned off during the landing. We used these binary data to confirm the other parameters' thresholds. With this set of parameters, we could identify flights longer than 18 s with a plateau phase longer than 8 s.
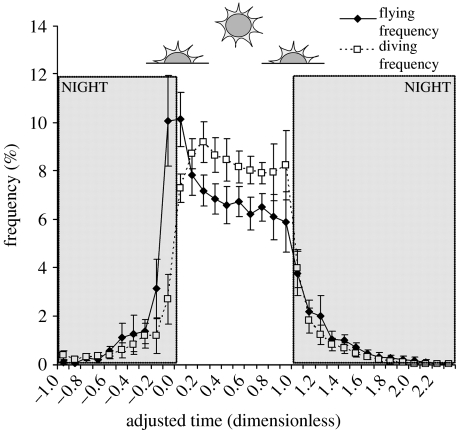
Local flights for each female were performed primarily between sunrise and sunset (morning=39±2% (s.e.m.) and afternoon=31±6%; figure 2). However, a substantial number of flights were also performed during the night (14±6%), at dawn (12±5%) and dusk (5±2%). Local flights were of higher frequency mainly 55 min either side of sunrise (20±4%), declined steadily throughout the day and experienced a rapid decline at dusk. Females were flying on average 9.6±2.4 min d−1 outside migration periods, i.e. only 0.7% of the day.
Figure 2.
Mean relative flying and diving frequencies (means±95% CI, %) recorded in 13 common eiders according to adjusted time of the day (where ‘0’ is sunrise and ‘1’ is sunset). These behaviours are performed outside the migration and moult periods from May to December 2003. See text for further details.
Diving activities were performed more during the day (morning=42±5% and afternoon=40±6%) than during other periods (night=10±6%, dusk=5±2% and dawn=3±3%). As a result, the average flight and dive frequencies were highly correlated (Pearson's r=0.88, permutation test p<0.001; figure 2). The diving frequency reached its maximum value approximately 2 h after sunrise.
4. Discussion
In the present study, we provide, to our knowledge, the first identification of all flights and foraging events in a wild bird. It is easy to distinguish flights (duration longer than 18 s) from other activities using fH data recorded by a DL in a bird performing forward flapping flight. The only activity that could be similar to flight is bathing behaviour, during which the bird is flapping its wings continuously and rapidly over the water and frequently submerging its head underwater. However, bathing behaviour, in spite of high mean fH, shows distinctive traces of fH such as a bell shape (without abrupt ascending and descending slopes of fH; figure 1c) with much variation in fH. This variation is caused by momentary bradycardia that occurs each time the bird submerges its head (Stephenson et al. 1986). Moreover, Ely et al. (1999) observed that antagonistic social interactions between geese (Anser albifrons) could rapidly increase heart rate to 400 beats per minute within a few seconds (1–2.5 s). However, since we considered only heart rate events longer than 18 s as a flight, we most probably excluded these types of behaviours.
Our results illustrate that outside of their migration periods, eider ducks fly for a very small proportion of the day. Flight frequency is particularly high around sunrise, decreases during the day and drops markedly during the night (figure 2). This pattern is matched, as hypothesized, by diving frequency. Common eiders are benthic predators, feeding mostly on blue mussels (Mytilus edulis), a sessile prey (Guillemette et al. 1992). As they feed little during the night (figure 2), they are probably drifting on the sea surface away from the food patches. As the starvation level increases throughout the night, hunger is at its highest level in the morning forcing the birds to forage again. Since coastal currents are tightly related to winds and tide levels, drifting away from the food patches during resting bouts (Guillemette et al. 1992) may explain the match between flight and diving frequency observed during day and night.
A detailed analysis of this relationship is beyond the scope of this preliminary report. For instance, in another contribution, we test the hypothesis that dive frequency between flights follows a lognormal distribution with a high frequency of dives right after a flight followed by much lower foraging activity before the next flight. Such a distribution of dive frequency is expected when flight costs are excessive (Pelletier 2006), which can be exacerbated by the accumulation of prey in the digestive system (Guillemette 1994). Nevertheless, we suggest here that flight behaviour of common eiders is an integral component of their foraging strategy.
Acknowledgments
All surgical procedures were conducted indoors on Christiansø island (Denmark) by a veterinary surgeon under a licence from Dyreforsøgtilsynet (Royal Veterinarian Corporation) and all birds were cared for in accordance to the principles and guidelines of the Canadian Council on Animal Care.
We thank Dr Annette Flagstad for performing all surgical procedures, Peter Lyngs and Yves Rigou for their help in the field, and Torben and Emma Jørgensen for their collaboration with the surgery. We also thank Joël Bêty, Jacques Larochelle and Laura McKinnon and four anonymous reviewers for their comments on this manuscript. This study was supported by discovery and equipment grants from the Canadian Natural Sciences and Engineering Research Council of Canada to M.G. and graduate fellowship to D.P.
Supplementary Material
Heart rate (means±95% CI, beats per minute) of common eiders engaged in 11 different behaviours (332 recordings, 4 to 86 recordings per behaviour). Asterisks indicate the statistical difference between flight and other activities (ANOVA—Tukey; *: p<0.02, ***: p<0.001 and n.s.: p>0.05). Values in parentheses are number of individuals observed per behaviour
References
- Ackerman J.T, Takekawa J.Y, Orthmeyer D.L, Fleskes J.P, Yee J.L, Kruse K.L. Spatial use by wintering greater white-fronted geese relative to a decade of habitat change in California's Central Valley. J. Wildl. Manage. 2006;70:965–976. doi:10.2193/0022-541X(2006)70[965:SUBWGW]2.0.CO;2 [Google Scholar]
- Bevan R.M, Boyd I.L, Butler P.J, Reid K, Woakes A.J, Croxall J.P. Heart rates and abdominal temperatures of free-ranging South Georgian shags, Phalacrocorax georgianus. J. Exp. Biol. 1997;200:661–675. doi: 10.1242/jeb.200.4.661. [DOI] [PubMed] [Google Scholar]
- Butler P.J, Woakes A.J, Bishop C.M. Behaviour and physiology of Svalbard barnacle geese Branta leucopsis during their autumn migration. J. Avian Biol. 1998;29:536–545. doi:10.2307/3677173 [Google Scholar]
- Casement M.B. Migration across the Mediterranean observed by radars. Ibis. 1966;108:461–491. [Google Scholar]
- Ely C.R, Ward D.H, Bollinger K.S. Behavioral correlates of heart rates of free-living greater white-fronted geese. Condor. 1999;101:390–395. doi:10.2307/1370002 [Google Scholar]
- Guillemette M. Digestive-rate constraint in wintering common eiders (Somateria mollissima): implications for flying capabilities. Auk. 1994;111:900–909. [Google Scholar]
- Guillemette M, Ydenberg R.C, Himmelman J.H. The role of energy intake rate in prey and habitat selection of common eiders Somateria mollissima in winter: a risk-sensitive interpretation. J. Anim. Ecol. 1992;61:599–610. doi:10.2307/5615 [Google Scholar]
- Guillemette M, Woakes A.J, Flagstad A, Butler P.J. Effects of data-loggers implanted for a full year in female common eiders. Condor. 2002;104:448–452. doi:10.1650/0010-5422(2002)104[0448:EODLIF]2.0.CO;2 [Google Scholar]
- Hays G.C, Åkesson S, Godley B.J, Luschi P, Santidrian P. The implications of location accuracy for the interpretation of satellite-tracking data. Anim. Behav. 2001;61:1035–1040. doi:10.1006/anbe.2001.1685 [Google Scholar]
- Kanwisher J.W, Williams T.C, Teal J.M, Lawson K.O., Jr Radiotelemetry of heart rates from free-ranging gulls. Auk. 1978;95:288–293. [Google Scholar]
- Lord R.D, Jr, Bellrose F.C, Cochran W.W. Radiotelemetry of the respiration of a flying duck. Science. 1962;137:39–40. doi: 10.1126/science.137.3523.39. doi:10.1126/science.137.3523.39 [DOI] [PubMed] [Google Scholar]
- Pelletier, D. 2006 Étude à long terme du comportement et de L'énergétique du vol chez L'Eider à duvet (Somateria mollissima) en millieu naturel. MSc thesis, Université du. Quebec à Rimouski.
- Pomeroy D. Why fly? The possible benefits for lower mortality. Biol. J. Linn. Soc. 1990;40:53–65. [Google Scholar]
- Stephenson R, Butler P.J, Woakes A.J. Diving behaviour and heart rate in tufted ducks (Aythya fuligula) J. Exp. Biol. 1986;126:341–359. doi: 10.1242/jeb.126.1.341. [DOI] [PubMed] [Google Scholar]
- Walsberg G.E. Avian ecological energetics. In: Farner D.S, King J.R, editors. Avian biology. Academic Press; New York, NY: 1983. pp. 161–220. [Google Scholar]
- Woakes A.J, Butler P.J, Bevan R.M. Implantable data logging system for heart rate and body temperature: its application to the estimation of field metabolic rates in Antarctic predators. Med. Biol. Eng. Comput. 1995;33:145–151. doi: 10.1007/BF02523032. doi:10.1007/BF02523032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heart rate (means±95% CI, beats per minute) of common eiders engaged in 11 different behaviours (332 recordings, 4 to 86 recordings per behaviour). Asterisks indicate the statistical difference between flight and other activities (ANOVA—Tukey; *: p<0.02, ***: p<0.001 and n.s.: p>0.05). Values in parentheses are number of individuals observed per behaviour