Abstract
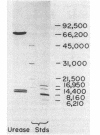
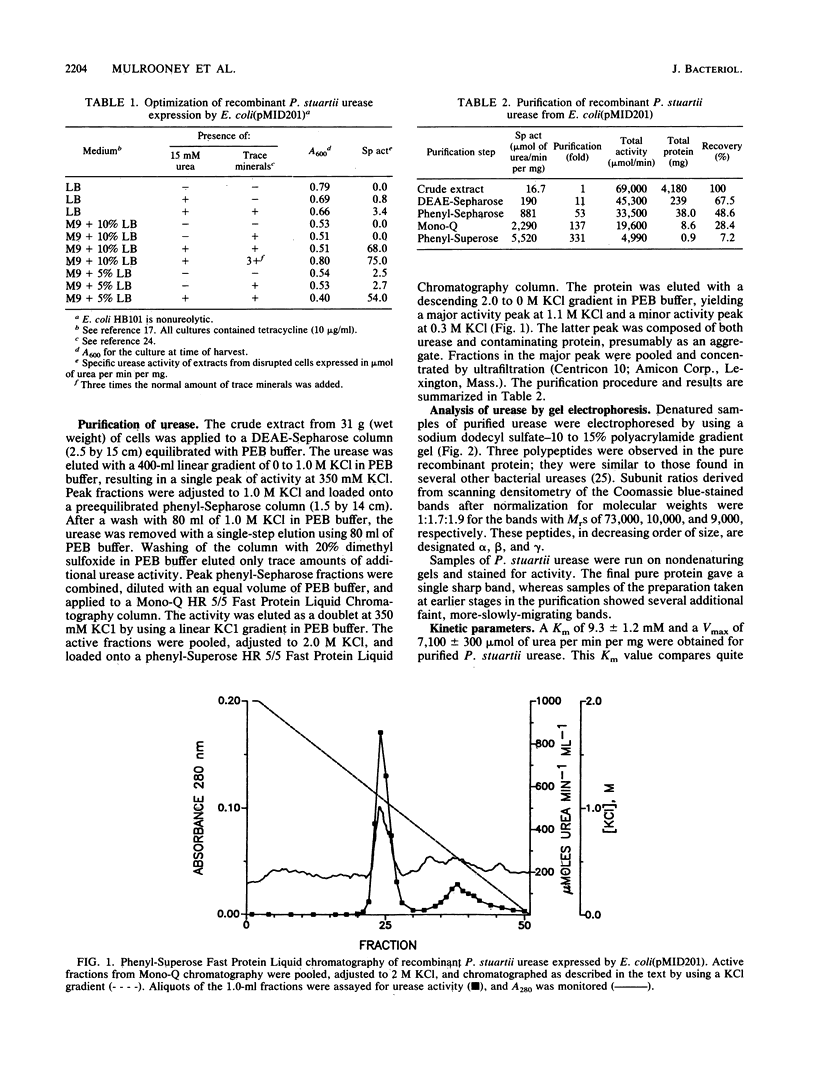
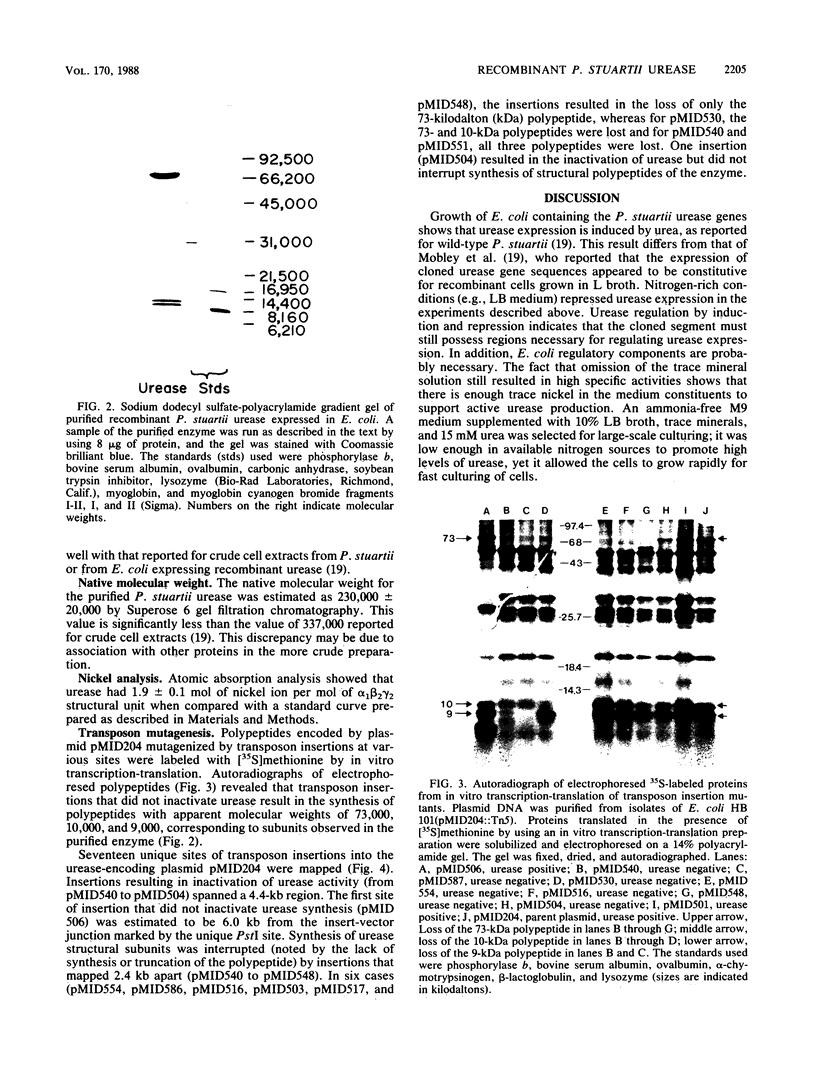
Recombinant urease from Providencia stuartii has been expressed in and purified from Escherichia coli, and the genetic organization of the structural genes has been determined. Urease expression was induced by urea and repressed by nitrogen-rich components in the medium. The urease protein was purified 331-fold by DEAE-Sepharose, phenyl-Sepharose, Mono-Q, and phenyl-Superose chromatographies with a 7.3% yield. The enzyme possessed a Km for urea of 9.3 mM and hydrolyzed urea at a Vmax of 7,100 mumol/min per mg. P. stuartii urease is composed of three polypeptides (Mrs, 73,000, 10,0000, and 9,000) denoted by alpha, beta, and gamma. The native enzyme is best described as (alpha 1 beta 2 gamma 2)2, based on a native Mr of 230,000, obtained by gel filtration chromatography, and on the Coomassie blue staining intensities of the individual subunits. Atomic absorption analysis of the pure protein revealed 1.9 +/- 0.1 nickel ions per alpha 1 beta 2 gamma 2 unit. In vitro transcription-translation analysis of transposon insertion mutants of the recombinant urease demonstrated that the urease peptides are encoded on adjacent DNA sequences and transcribed as a polycistronic mRNA in the order gamma, beta, and then alpha. Three urease-defective insertion mutants were identified that did not affect synthesis of urease subunit polypeptides, indicating that some nickel processing, enzyme activation, or other function may also be necessary for producing an active urease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. K., Blakeley R. L., Zerner B. Urea and urease. Adv Inorg Biochem. 1984;6:245–283. [PubMed] [Google Scholar]
- BRAUDE A. I., SIEMIENSKI J. Role of bacterial urease in experimental pyelonephritis. J Bacteriol. 1960 Aug;80:171–179. doi: 10.1128/jb.80.2.171-179.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattler D. P., Contaxis C. C., Reithel F. J. Dissociation of urease by glycol and glycerol. Nature. 1967 Oct 21;216(5112):274–275. doi: 10.1038/216274b0. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Hickman F. W., Brenner D. J., Schreiber M., Rickenbach D. G. Unusual Enterobacteriaceae. "Proteus rettgeri" that "change" into Providencia stuartii. J Clin Microbiol. 1977 Oct;6(4):373–378. doi: 10.1128/jcm.6.4.373-378.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R. B., Penner J. L., Hennessy J. N., Jackowski B. J. Transferable urease activity in Providencia stuartii. J Clin Microbiol. 1981 Mar;13(3):561–565. doi: 10.1128/jcm.13.3.561-565.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausinger R. P. Purification of a nickel-containing urease from the rumen anaerobe Selenomonas ruminantium. J Biol Chem. 1986 Jun 15;261(17):7866–7870. [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Genetic and biochemical diversity of ureases of Proteus, Providencia, and Morganella species isolated from urinary tract infection. Infect Immun. 1987 Sep;55(9):2198–2203. doi: 10.1128/iai.55.9.2198-2203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Fraiman M. H., Tenney J. H., Warren J. W. Variable phenotypes of Providencia stuartii due to plasmid-encoded traits. J Clin Microbiol. 1985 Nov;22(5):851–853. doi: 10.1128/jcm.22.5.851-853.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Jones B. D., Jerse A. E. Cloning of urease gene sequences from Providencia stuartii. Infect Immun. 1986 Oct;54(1):161–169. doi: 10.1128/iai.54.1.161-169.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Warren J. W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987 Nov;25(11):2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Hamilton-Miller J. M. Inhibitors of urease as chemotherapeutic agents. Crit Rev Microbiol. 1984;11(1):1–12. doi: 10.3109/10408418409105901. [DOI] [PubMed] [Google Scholar]
- Samtoy B., DeBeukelaer M. M. Ammonia encephalopathy secondary to urinary tract infection with Proteus mirabilis. Pediatrics. 1980 Feb;65(2):294–297. [PubMed] [Google Scholar]
- Smith C. J., Hespell R. B., Bryant M. P. Ammonia assimilation and glutamate formation in the anaerobe Selenomonas ruminantium. J Bacteriol. 1980 Feb;141(2):593–602. doi: 10.1128/jb.141.2.593-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd M. J., Hausinger R. P. Purification and characterization of the nickel-containing multicomponent urease from Klebsiella aerogenes. J Biol Chem. 1987 May 5;262(13):5963–5967. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. W. Providencia stuartii: a common cause of antibiotic-resistant bacteriuria in patients with long-term indwelling catheters. Rev Infect Dis. 1986 Jan-Feb;8(1):61–67. doi: 10.1093/clinids/8.1.61. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]