Abstract
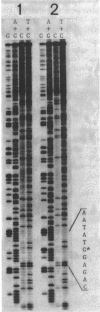
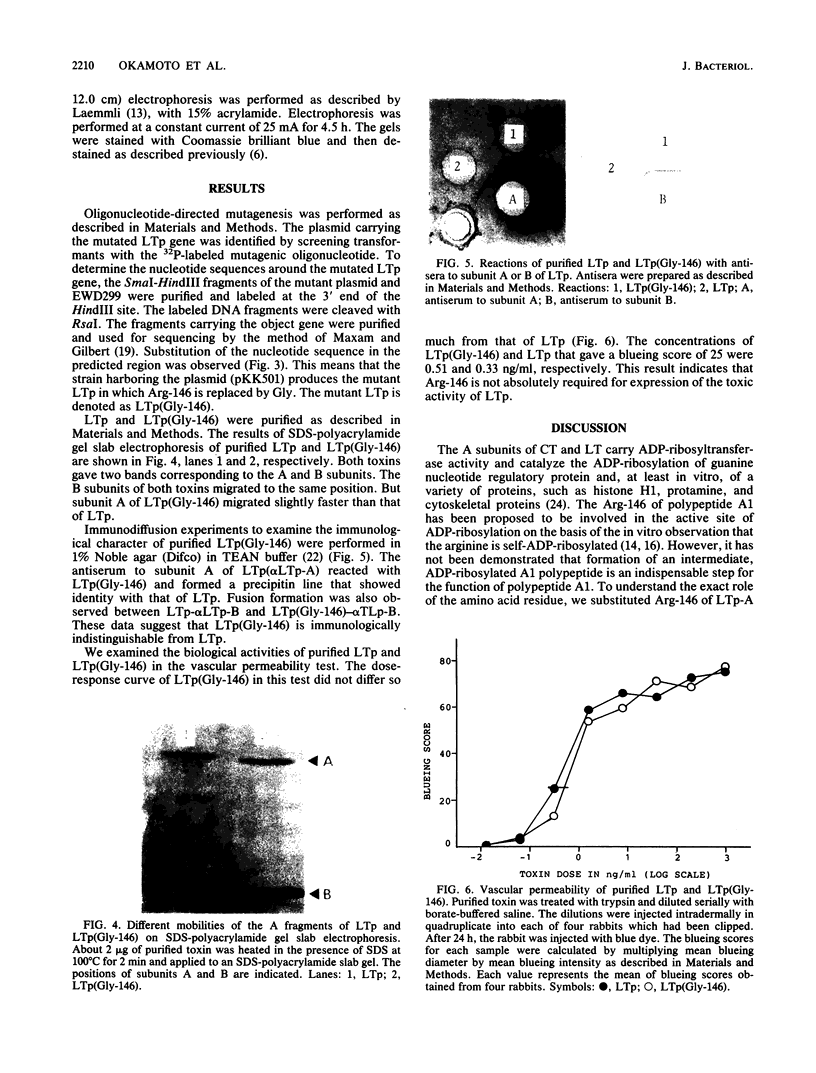
The ADP-ribosyltransferase activity of polypeptide A1 of cholera toxin and that of Escherichia coli heat-labile enterotoxin (LT) are primarily responsible for the toxic activities of these toxins. Since the amino acid sequences of the two A1 polypeptides are very similar, their functional mechanisms are considered to be the same. Arg-146 of polypeptide A1 is thought to be involved in the active site, because this amino acid of cholera toxin has been identified as the site of self-ADP-ribosylation. However, the exact role of Arg-146 and the significance of self-ADP-ribosylation in toxicity remain unclear. We substituted Arg-146 of polypeptide A1 of LT with Gly by oligonucleotide-directed mutagenesis and examined the biological property of the resultant mutant LT. The substitution changed the mobility of subunit A on sodium dodecyl sulfate-polyacrylamide gel but did not reduce the vascular permeability activity of LT. This result indicates that Arg-146 is not absolutely required for toxic activity and that LT can express its toxic activity without self-ADP-ribosylation at Arg-146.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., Shortle D. Strategies and applications of in vitro mutagenesis. Science. 1985 Sep 20;229(4719):1193–1201. doi: 10.1126/science.2994214. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Immunological cross-reactivity between a heat-labile enterotoxin(s) of Escherichia coli and subunits of Vibrio cholerae enterotoxin. Infect Immun. 1978 Sep;21(3):1036–1039. doi: 10.1128/iai.21.3.1036-1039.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P., Yamamoto K., Takeda Y., Miwatani T. Production of cholera-like enterotoxin by a Vibrio cholerae non-O1 strain isolated from the environment. Infect Immun. 1981 Oct;34(1):90–97. doi: 10.1128/iai.34.1.90-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Gill D. M., Falkow S. Cistrons encoding Escherichia coli heat-labile toxin. J Bacteriol. 1979 Sep;139(3):850–858. doi: 10.1128/jb.139.3.850-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Clements J. D., Robertson D. C., Finkelstein R. A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Sep;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Honda T., Takeda Y., Miwatani T. Isolation of special antibodies which react only with homologous enterotoxins from Vibrio cholerae and Enterotoxigenic Escherichia coli. Infect Immun. 1981 Nov;34(2):333–336. doi: 10.1128/iai.34.2.333-336.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. Y. Bacterial protein toxins with latent ADP-ribosyl transferases activities. Adv Enzymol Relat Areas Mol Biol. 1986;58:99–140. doi: 10.1002/9780470123041.ch3. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Cancedda F., Duffy L. K. ADP-ribosyl transferase activity of cholera toxin polypeptide A1 and the effect of limited trypsinolysis. Biochem Biophys Res Commun. 1981 Oct 15;102(3):1021–1027. doi: 10.1016/0006-291x(81)91640-5. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Xia Q. C., Salotra P. T. Location and amino acid sequence around the ADP-ribosylation site in the cholera toxin active subunit A1. Biochem Biophys Res Commun. 1983 Oct 14;116(1):341–348. doi: 10.1016/0006-291x(83)90420-5. [DOI] [PubMed] [Google Scholar]
- Lockman H. A., Galen J. E., Kaper J. B. Vibrio cholerae enterotoxin genes: nucleotide sequence analysis of DNA encoding ADP-ribosyltransferase. J Bacteriol. 1984 Sep;159(3):1086–1089. doi: 10.1128/jb.159.3.1086-1089.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Honda T., Sima H., Tsuji T., Miwatani T. Analysis of antigenic determinants in cholera enterotoxin and heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1983 Jul;41(1):50–53. doi: 10.1128/iai.41.1.50-53.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T., Honda T., Miwatani T., Wakabayashi S., Matsubara H. Analysis of receptor-binding site in Escherichia coli enterotoxin. J Biol Chem. 1985 Jul 15;260(14):8552–8558. [PubMed] [Google Scholar]
- Tsuji T., Taga S., Honda T., Takeda Y., Miwatani T. Molecular heterogeneity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1982 Nov;38(2):444–448. doi: 10.1128/iai.38.2.444-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Vlasuk G. P., Inouye S., Ito H., Itakura K., Inouye M. Effects of the complete removal of basic amino acid residues from the signal peptide on secretion of lipoprotein in Escherichia coli. J Biol Chem. 1983 Jun 10;258(11):7141–7148. [PubMed] [Google Scholar]
- Yamamoto T., Nakazawa T., Miyata T., Kaji A., Yokota T. Evolution and structure of two ADP-ribosylation enterotoxins, Escherichia coli heat-labile toxin and cholera toxin. FEBS Lett. 1984 Apr 24;169(2):241–246. doi: 10.1016/0014-5793(84)80326-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tamura T., Yokota T. Primary structure of heat-labile enterotoxin produced by Escherichia coli pathogenic for humans. J Biol Chem. 1984 Apr 25;259(8):5037–5044. [PubMed] [Google Scholar]