Abstract
In this study the role of myeloperoxidase (MPO) in a murine (C57BL/6) model of ischemia and reperfusion (I/R)-induced renal failure was investigated. The renal function after I/R was analyzed in MPO-deficient (Mpo−/−) mice and compared with wild-type (WT) controls. A significant reduction in renal function loss (blood urea nitrogen) was observed after 24 hours of reperfusion of ischemically damaged kidneys in Mpo−/− mice compared with I/R WT controls (I/R Mpo−/− = 31.3 ± 1.7 mmol/L versus I/R WT = 42.8 ± 2.1 mmol/L, sham = 7.0 ± 0.5 mmol/L; P = 0.003). The early reperfusion phase (2 hours of reperfusion) was characterized by a substantial increase in apoptosis and early complement activation, surprisingly similar in Mpo−/− and WT mice. Improved renal function in Mpo−/− mice after extended reperfusion was accompanied by a reduced neutrophil influx (P = 0.017) compared with WT controls. Activation and deposition of complement was not significantly reduced in Mpo−/− mice compared with WT controls after 24 hours of reperfusion, indicating no specific in vivo role for MPO in activating complement after renal I/R. Taken together, these results demonstrated an important contribution of MPO in the induction of organ damage after renal I/R by influencing critical factors such as neutrophil extravasation but not complement activation.
In clinical medicine, complications arising from organ ischemia and reperfusion (I/R) are common phenomena, complicating the treatment of severely injured or ill patients and influencing the outcome of various clinical conditions. Throughout the past decades studies into the pathophysiology of I/R-induced organ damage have identified several critical factors—cellular injury, endothelial dysfunction, microcirculatory collapse, neutrophil activation and extravasation, and complement activation, all contributing to the development of organ dysfunction. Our limited understanding of exact pathophysiological mechanisms has so far impaired the development of new and effective therapies.
Cellular injury, induced by ischemia and aggravated on reperfusion, forms a potent trigger for activation of an extensive inflammatory response, illustrated by the production of various cytokines such as tumor necrosis factor-α, interferon-γ, and the interleukins 6, 10, 12, and 18,1,2 the activation and sequestration of polymorphonuclear neutrophils (PMNs) in the affected area,3 as well as the expression and deposition of various components of the innate immune response, such as complement factors.4,5,6 Under healthy conditions, cells and proteins of the innate immune system protect the organism by orchestrating a well mounted attack on invading microorganisms, but when faced with extensive I/R injury, sufficient means of control seem absent. Already during the 1980s a detrimental role for PMNs was shown during hypoperfusion and ischemia followed by reperfusion.7,8 These early studies proposed a role for activated neutrophils in the frequently observed no-reflow phenomenon9 and the generation of harmful reactive oxygen species after reperfusion of ischemically damaged tissue.10
Myeloperoxidase (MPO) is a 140-kDa heme protein that is predominantly stored in the lysosomes of monocytes and in the azurophilic granules of PMNs.11 It is one of the most abundant enzymes released on neutrophil activation. The capacity of MPO to catalyze the formation of hypochlorite (HOCl) from hydrogen peroxide (H2O2) and chloride ions makes it a powerful tool in the bactericidal armament of these cells. However, there are clinical studies indicating a potentially harmful effect of MPO in immune-mediated inflammatory syndromes, such as multiple sclerosis,12 acute coronary syndrome,13,14 and renal disease.15 In addition, a considerable line of research indicates that MPO and MPO-derived oxidants are involved in the pathogenesis of atherosclerosis,16,17,18 organ damage after myocardial I/R and infarction,19 as well as complement activation in vitro.20 Furthermore, MPO contributes to the dysfunction of the local vasculature during acute inflammation by modifying local NO production and availability.21
To study the in vivo contribution of MPO in the development of I/R-induced injury, the role of MPO in a mouse model of renal I/R injury was investigated. Disease severity was compared between MPO-deficient (Mpo−/−) and wild-type (WT) mice with respect to renal function, complement activation, neutrophil activation and extravasation, renal morphology, and apoptosis. Our results show that MPO plays a detrimental role in the pathogenic mechanisms involved in this model and is in part responsible for the development of renal damage resulting from I/R without influencing complement activation in vivo.
Materials and Methods
Mice
Mpo−/− mice, backcrossed to a C57BL/6 background six times, were genotyped using polymerase chain reaction (PCR)-amplified DNA from tail clippings.22 WT male C57BL/6 control mice (11 weeks of age) were obtained from Charles River Breeding Laboratories (Heidelberg, Germany). Mice were kept according to University of Maastricht animal facility regulations, and all experiments were approved by the local animal ethical committee.
Experimental Procedures
Experiments were performed as previously described, with minor modifications.6 At the start of the experiments, mice (n = 6 in each group) were anesthetized with sodium pentobarbital (100 mg/kg i.p.). Body temperature was maintained at 37°C by a heating pad until animals recovered from anesthesia. Under aseptic conditions a 1.0-cm-long midline abdominal incision was made, and ischemia was induced by applying a nontraumatic vascular clamp to the left renal pedicle for 40 minutes. Subsequently, the wound was covered with cotton soaked in sterile phosphate-buffered saline (PBS). After removal of the clamp, the left kidney was inspected for restoration of blood flow and the contralateral kidney was removed and stored for analysis. The wound was closed in two layers and in mice that were to be sacrificed after 24 hours, 0.25% bupivacaine was applied topically to the wound for postoperative pain management. The animals were sacrificed 2 or 24 hours after reperfusion. At the time of sacrifice, plasma was collected and the left kidney was harvested for morphological, immunohistochemical, and immunofluorescent analyses. Macroscopic evaluation of the ischemic kidneys during the procedure resulted in the exclusion of one mouse, because its kidney appeared to have been insufficiently ischemic during the experiment. One WT frozen tissue sample to be used for preparation of frozen sections was lost during work-up.
Plasma Measurements of Blood Urea Nitrogen (BUN) and MPO
In mice sacrificed after 24 hours, BUN levels in the plasma were determined by an enzymatic degradation assay on a Synchron LX20 PRO chemistry analyzer (Beckman Coulter Inc., Fullerton, CA). Plasma MPO levels were determined by in-house catching enzyme-linked immunosorbent assay as described previously.23 Briefly, microtiter plates were coated (1 μg/ml) with Fcγ fragment-specific goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA), incubated for 48 hours at 4°C, and blocked with 1% bovine serum albumin in PBS. Plates were then incubated with an anti-murine MPO-specific monoclonal antibody (mAb) (clone 8F4; Hbt, Uden, The Netherlands), followed by incubation with appropriately diluted plasma samples. Next, the plates were incubated with polyclonal rabbit anti-human MPO (DakoCytomation, Glostrup, Denmark) and alkaline phosphatase-labeled polyclonal goat anti-rabbit IgG as primary and secondary detection antibody, respectively. 4-Nitrophenyl phosphate (pNPP) was used as substrate, and results were analyzed spectrophotometrically at 405 nm. Concentrations were calculated from a standard curve of purified murine MPO (range, 2.5 to 100 ng/ml).
Immunofluorescence and Immunohistochemistry
Kidneys were snap-frozen in OCT compound. Five-μm sections were cut with a cryostat, dried, and stored at −70°C. The slides were fixed in −20°C cold acetone and stained for neutrophil influx using a rat anti-mouse neutrophil antibody (clone NIMP-R14, Hbt).24 After incubation of the primary antibody, endogenous peroxidase activity was blocked using 0.05% H2O2 in PBS, and rabbit anti-rat IgG and goat anti-rabbit IgG-PO (both DakoCytomation) were then used as secondary and tertiary antibodies, respectively. Antibody binding was visualized using 3-amino-9-ethylcarbazole with H2O2 as substrate. Sections were counterstained with hematoxylin, and juxtamedullary neutrophil influx was quantified by counting the average number of NIMP-R14-positive cells in 10 high-power fields of four tissue sections per kidney in WT and Mpo−/− mice (n = 5 per group), at 24 hours after reperfusion.
To determine co-localization of PMNs with renal MPO deposits, renal cross-sections were double-stained with a biotinylated mouse mAb specific for murine MPO (clone 8F4, Hbt)23 and rat anti-mouse neutrophil mAb NIMP-R14 (Hbt), using Alexa 488-labeled streptavidin and Alexa 568-labeled goat anti-rat IgG (both Invitrogen Molecular Probes, Leiden, The Netherlands), respectively, as conjugates.
Murine complement factors MBL-A, MBL-C, C3, C6, and C9 were determined by using rat monoclonal (MBL-A, clone 8G6; MBL-C, clone 14D12; iC3b, clone 2/11; Hbt) or rabbit polyclonal anti-mouse C6 (kindly provided by Dr. A. Tenner, University of California, Irvine, CA) and rabbit anti-mouse C9 (in-house made by M.R.D.) primary antibodies. Specific binding was detected by using peroxidase-conjugated secondary antibodies to rat and rabbit IgG, respectively (Jackson ImmunoResearch). Staining was visualized by 3-amino-9-ethylcarbazole followed by hematoxylin. No significant staining was detected in slides incubated with control rat IgG (for NIMP-R14, MBL-A, MBL-C, and C3), mouse IgG (for MPO), and rabbit IgG (for C6 and C9). After immunohistochemical staining of kidneys (n = 5 per group), renal deposition of complement factors was scored arbitrarily as negative (−), slightly positive (+), moderately positive (++), and intensively positive (+++).
Western Blot
Western blot analyses of C6 deposition in sham-treated or reperfused ischemic knockout (KO) and WT kidneys was performed as described before, with minor modifications.25 Renal tissue samples from WT and KO I/R or sham-treated animals were homogenized in lysis buffer (200 mmol/L NaCl, 10 mmol/L Tris base, 5 mmol/L ethylenediaminetetraacetic acid, 10% glycerin, 1 mmol/L phenylmethyl sulfonyl fluoride, 0.1 U/ml aprotinin, and 1 μg/ml leupeptin). Tissue homogenates were centrifuged at 300 rpm for 10 minutes, after which the collected supernatants were centrifuged again at 10,000 rpm for 3 minutes. The protein concentration of the different lysates was determined using Bradford analyses. Aliquots containing equal amounts (10 μg) of total protein or normal mouse serum as positive control were heated to 100°C for 5 minutes in sodium dodecyl sulfate-sample buffer containing 5% β-mercaptoethanol (Sigma, Chicago, IL), transferred to a 8% sodium dodecyl sulfate-polyacrylamide gel and blotted on an Immobilon-P polyvinylidene difluoride membrane (Millipore, Bedford, MA). After blotting of the proteins, the blocking and antibody incubation steps were performed in phosphate-buffered saline containing 5% bovine serum albumin and 0.1% Tween 20 (Sigma). C6 was detected by incubating polyvinylidene difluoride membranes overnight at 4°C in buffer containing properly diluted rabbit anti-mouse C6 (Hbt). Binding of the primary antibody was detected with a peroxidase-conjugated secondary antibody to rabbit IgG (Jackson ImmunoResearch). After washing positive bands were visualized using chemiluminescence (Supersignal; Pierce, Rockford, IL).
Apoptosis Assay
The presence of internucleosomal DNA cleavage in kidneys was established with a commercial ligase-mediated-PCR assay kit (Apoalert; Clontech, Palo Alto, CA), enabling semiquantitative measurement of the extent of apoptosis.26
Renal Morphology
Paraffin-embedded sections from the 24-hour reperfusion group were prepared and stained using periodic acid-Schiff staining. Morphological changes resulting from I/R injury were graded using the scoring system described by Leemans and colleagues.27 Tubules, cast deposition, brush border loss, and necrosis were identified in at least 10 randomly chosen ×200 fields in the cortico-medullary region of three sections per kidney. Total scores were calculated for each kidney.
Statistical Analysis
Data are expressed as means ± SEM and were analyzed by unpaired two-tailed Student’s t-test, using Graphpad Prism 4.01 for Windows (Graphpad Software, San Diego, CA). A P value ≤0.05 was considered statistically significant.
Results
Renal Histology
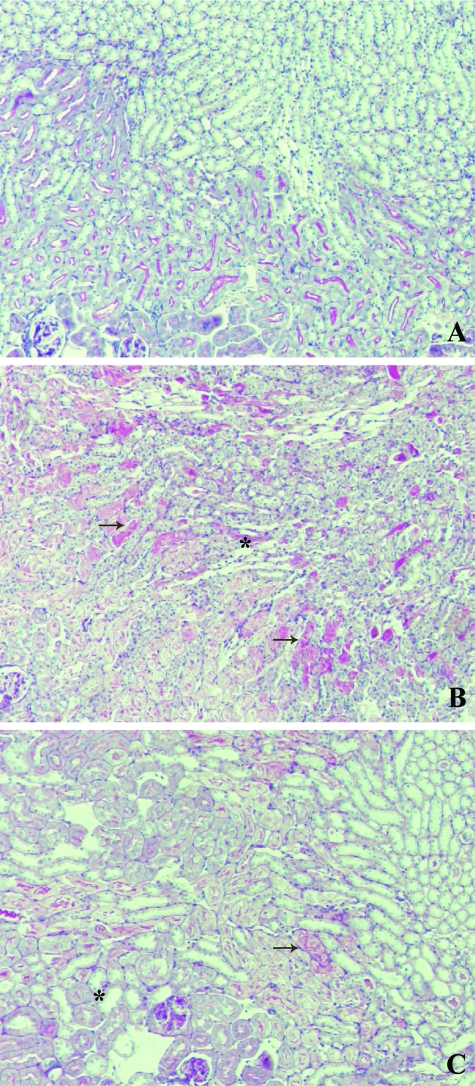
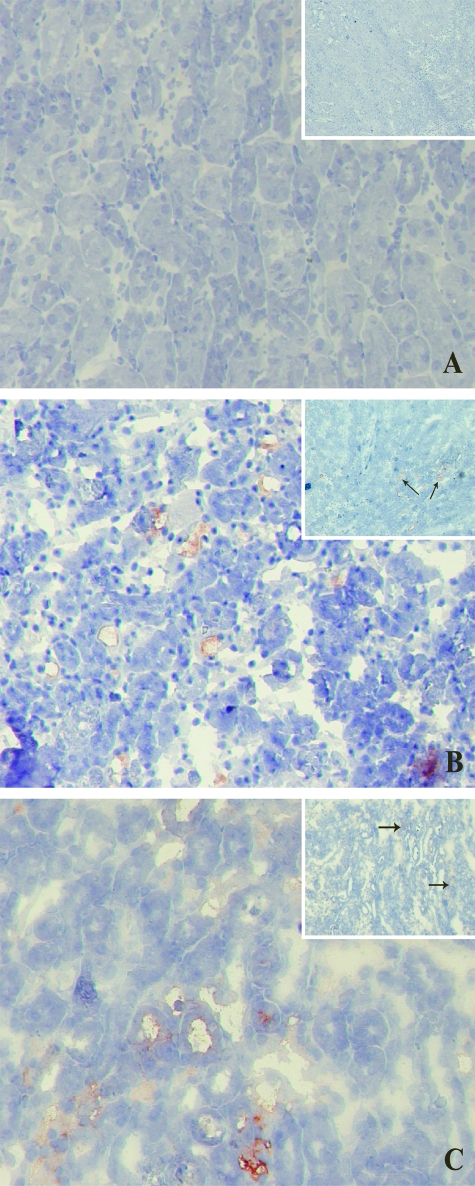
To directly assess tissue damage induced by 40 minutes of ischemia followed by 24 hours of reperfusion, paraffin sections were stained using periodic acid-Schiff staining (Figure 1). Moderate to severe damage involving ∼25% of the cortex was similarly observed in both Mpo−/− and WT kidneys (Table 1), using the histopathological scoring system developed by Leemans and colleagues27 to assess the renal damage. No damage was seen in sham-treated animals.
Figure 1.
Similar renal morphology of ischemically damaged WT and KO kidneys after 24 hours of reperfusion. Microphotographs showing periodic acid-Schiff staining of kidney sections of sham-treated (A) as well as WT (B) and Mpo−/− (C) mice subjected to 40 minutes of ischemia followed by 24 hours of reperfusion. Shown are representative microphotographs of all groups. Original magnifications, ×100.
Table 1.
Immunohistochemical and Histological Analysis of Renal Complement Deposition and Damage
| Ischemia (minutes) | Reperfusion (hours) | Lectin pathway
|
Alternative Pathway C3 | Common pathway C6/C9 | Histology PAS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MBL-A
|
MBL-C
|
WT | Mpo−/− | WT | Mpo−/− | WT | Mpo−/− | ||||
| WT | Mpo−/− | WT | Mpo−/− | ||||||||
| − | − | − | − | − | − | − | − | − | − | 0 | 0 |
| 40 | 2 | − | − | + | + | + | + | − | − | − | − |
| 40 | 24 | ++ | ++ | ++ | ++ | + | + | ++ | ++ | 29.2 | 27.4* |
Average score from 10 randomly chosen ×200 fields. I/R WT = 29.2 ± 2.5 versus I/R Mpo−/− = 27.4 ± 3.3, P = 0.68.
MPO Deficiency Reduces Renal Dysfunction
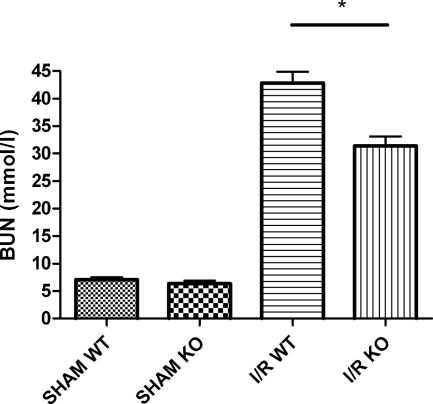
Renal dysfunction was reflected by an increase in BUN levels after 24 hours of reperfusion (Figure 2). Forty minutes of unilateral ischemia followed by 24 hours of reperfusion caused an elevation of BUN levels in Mpo−/− and WT mice. However, Mpo−/− mice displayed a markedly less pronounced increase in renal failure compared with WT mice (I/R Mpo−/− = 31.3 ± 1.7 mmol/L versus I/R WT = 42.8 ± 2.1 mmol/L, P = 0.003, sham = 7.0 ± 0.5 mmol/L). These findings illustrate a contribution of MPO to the development of organ failure of reperfused ischemic kidneys.
Figure 2.
MPO deficiency significantly reduces renal function loss after renal I/R. Renal function after 24 hours of reperfusion as reflected by BUN concentration. Statistical significance of renal function in Mpo−/− mice as compared with WT animals was denoted at P = 0.003 (*).
MPO Deficiency Fails to Abrogate Apoptosis and Mannose-Binding Lectin Deposition during Early Reperfusion
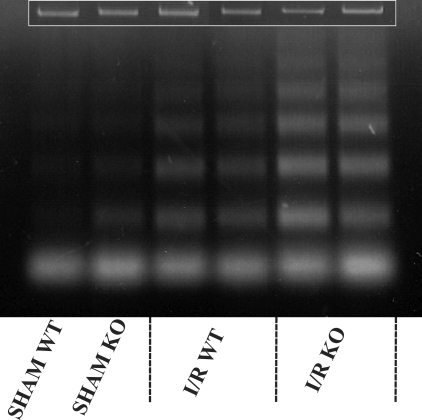
Previously, it was demonstrated that apoptosis plays an important role in the development of organ damage induced by the reperfusion of ischemic kidneys.28 Because some studies show that MPO induces apoptosis by directly mediating caspase activation, the hypothesis that MPO deficiency preserves renal function by inhibition of apoptosis was tested. Apoptosis after 2 hours of reperfusion, analyzed by typical DNA cleavage, is depicted in Figure 3. Clearly shown is the I/R-induced increase in apoptosis, when comparing experimental and sham-treated animals. Mpo−/− and WT animals showed a similar increase in apoptosis. The slightly larger amount of apoptosis that was observed in Mpo−/− as compared with WT mice after 2 hours of reperfusion, was similarly observed in sham-treated Mpo−/− and WT mice. This indicates that the rise in apoptosis in Mpo−/− and WT experimental animals was in fact similar and certainly not reduced in Mpo−/− animals.
Figure 3.
MPO deficiency does not affect I/R-induced early apoptosis. Shown are two sham-treated animals as well as two representatives of each experimental group. Loading controls are given in the inset.
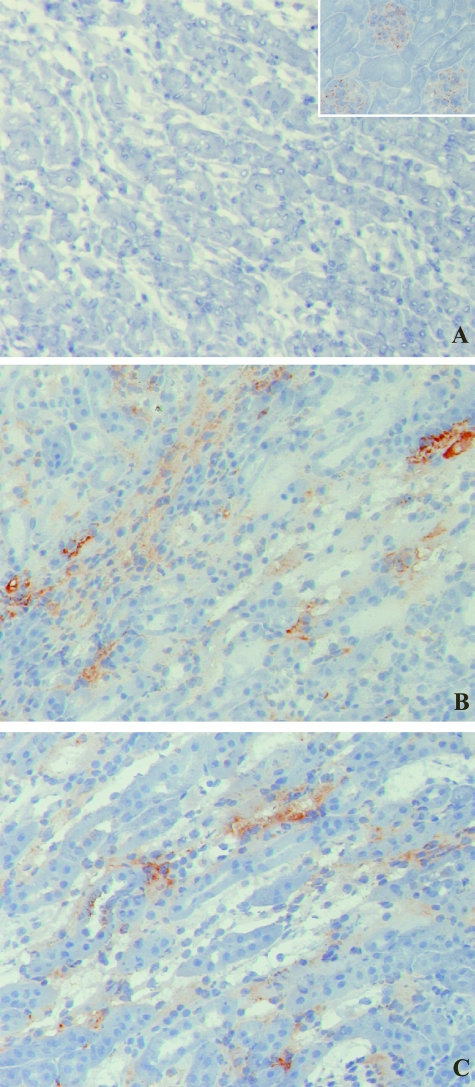
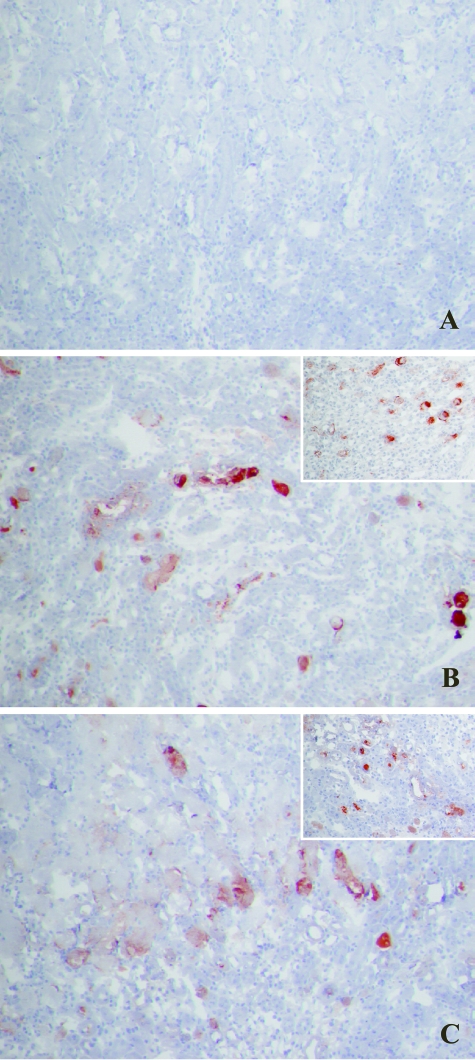
It has been shown that bound complement components are critical in the induction and propagation of I/R-induced organ damage after initial cellular damage and apoptosis.29,30 Predominantly the murine MBL variant C is one of the first inflammatory factors observed to bind during early reperfusion.31 Therefore, immunohistochemical staining was performed on early 2-hour reperfusion samples, to study the deposition of activated MBL-C. No differences were detected in the amount of MBL-C present in the cortico-medullary region on peritubular capillaries and in the interstitium of reperfused ischemic kidneys from either Mpo−/− or WT mice (Figure 4), suggesting similar levels of complement activation as a result of comparable ischemia-induced cellular damage and apoptosis in Mpo−/− and WT animals.
Figure 4.
Two hours of reperfusion of ischemically damaged kidneys results in early MBL-C deposition, similar in Mpo−/− and WT mice. MBL-C binding in WT sham-treated (not shown) and KO sham-treated (A) animals was only observed in glomeruli. MBL-C deposition was evident in peritubular capillaries, the interstitium, and along the epithelial brush border of damaged tubules in both WT (B) and Mpo−/− (C) mice subjected to renal I/R. Shown are representative microphotographs of all groups. Original magnifications: ×200; ×600 (inset).
PMN Influx and MPO Release
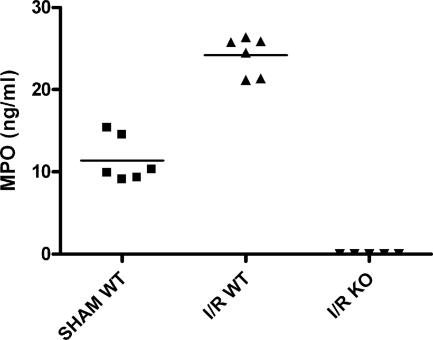
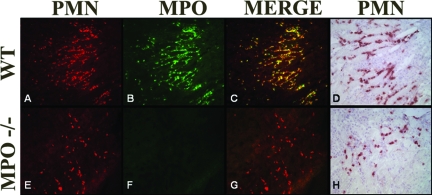
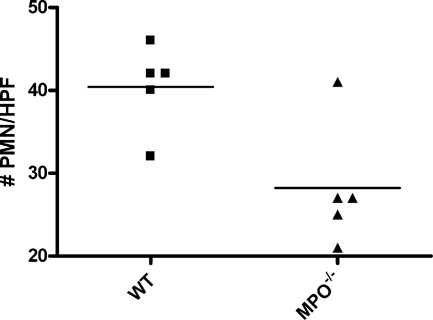
Next the influx and activation of inflammatory mediators present during the subsequent progression phase of reperfusion was investigated. Neutrophils invading the damaged tissue contribute to the local inflammatory response in part by releasing their lysosomal constituents, including MPO. MPO released by the activated PMN has been shown to be important in the activation and adhesion of other neutrophils.32,33 WT mice had high levels of circulating MPO (Figure 5), indicating I/R-mediated neutrophil activation. This idea was strengthened by the lack of MPO in sham-treated animals. Immunohistology revealed high levels of MPO in the kidney, mostly comprised to NIMP-R14-positive PMNs and their direct surroundings (Figure 6, A–C). As expected MPO was absent in samples from Mpo−/− mice (Figures 5 and 6F). To quantify the neutrophil infiltration, we counted cells positive for NIMP-R14, a neutrophil marker not influenced by the lack of MPO. Analysis revealed a significant reduction of I/R-induced PMN infiltration of the Mpo−/− kidneys in comparison to the WT (I/R Mpo−/− = 28.2 ± 3.3 PMN/high-power fields versus I/R WT = 40.4 ± 2.3 PMN/high-power fields, P = 0.017), indicating an important in vivo role for MPO in the extravasation of neutrophils after I/R (Figure 7).
Figure 5.
MPO plasma levels, representing total neutrophil activation, are increased 24 hours after reperfusion of ischemically damaged WT kidneys. No MPO was detected in Mpo−/− mice. Statistical significance of WT MPO levels as compared with sham-treated animals was denoted at P < 0.0001.
Figure 6.
Immunofluorescent staining showing PMN sequestration (red, Alexa 568) and MPO presence (green, Alexa 488) in kidneys of WT (A–C) and Mpo−/− (E–G) mice subjected to I/R. Overlapping PMN and MPO data depict the fact that MPO is predominantly located around NIMP-R14-positive PMNs. D (WT) and H (Mpo−/−) show PMN infiltration in relation to structural changes. Shown are representative microphotographs of all groups. Original magnifications, ×200.
Figure 7.
MPO deficiency reduces neutrophil infiltration after renal I/R. Statistical significance as compared with WT animals was denoted at P = 0.017.
Complement Activation
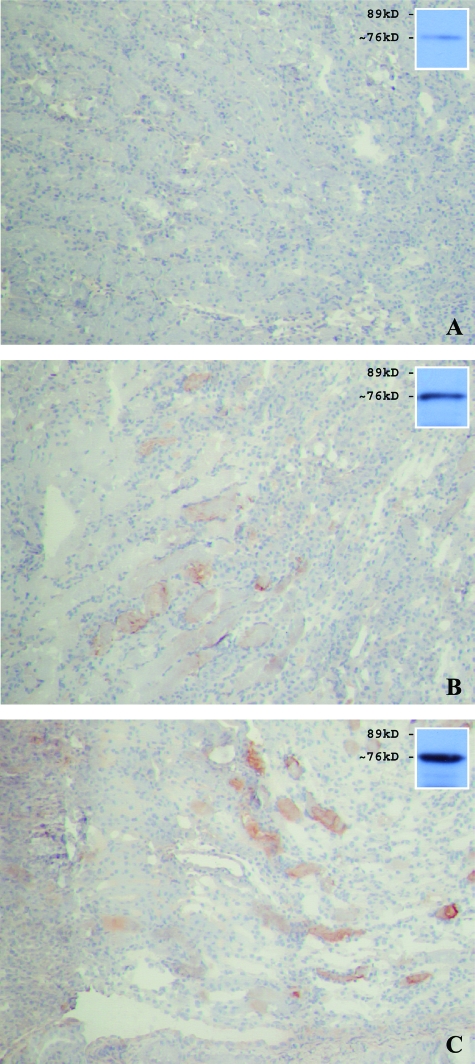
Studies of the complement system during renal I/R revealed the lectin and alternative as well as the subsequent common complement pathways as key effectors in the induction of I/R-induced organ failure.4,31 MPO has been described as an activator of complement.20 In vitro experiments revealed complement component C5 activating properties for various proteolytic enzymes that were released on PMN stimulation. Similarly, purified MPO was shown to activate C5 by hypochlorite formation in vitro. From this we hypothesized that MPO might regulate complement activation and influence renal function in an in vivo renal I/R model. Activation of the complement system was assessed after prolonged 24 hours of reperfusion (Table 1). Immunohistochemistry revealed similar activation of early and late common pathway complement proteins in Mpo−/− and WT mice. The deposition of early complement factors MBL-A, MBL-C, and C3 increased in both Mpo−/− and WT mice after 24 hours of reperfusion when compared with the 2-hour reperfusion samples. MBL-C and C3 deposition was abundantly present in peritubular capillaries and interstitium as well as on the epithelial lining of damaged tubules after I/R. Surprisingly, no differences in the quantity of complement proteins MBL-A, MBL-C, and C3 were detected between Mpo−/− and WT groups subjected to I/R (Figure 8). Because C5 convertase-like properties have been described for MPO in vitro, the in vivo deposition of common pathway complement components was analyzed. Similar to the results observed for early complement proteins, the deposition of common pathway proteins C6 (Figure 9), analyzed by immunohistochemical staining and Western blot, and C9 (Figure 10) were not substantially reduced in MPO-deficient mice after 24 hours of reperfusion, suggesting no considerable role for MPO in the activation of common pathway complement components in vivo in response to kidney I/R. C6 and C9 depositions were similarly observed in WT and Mpo−/− mice on the epithelial lining of damaged tubules as well as on tubular cast formations observed after 24 hours of reperfusion.
Figure 8.
MBL-C and C3 (insets) deposition in WT and Mpo−/− mice after 24 hours of reperfusion. MBL-C and C3 deposition was not different in WT (B) or Mpo−/− (C) mice. A: Virtually no MBL-C or C3 staining was detected in sham-treated WT (not shown) and Mpo−/− mice. Corresponding data were obtained for MBL-A deposition (data not shown). Shown are representative microphotographs of all groups. Original magnifications: ×200; ×100 (insets).
Figure 9.
Common pathway activation as determined by immunohistochemical and Western blot analyses of C6 deposition in WT and Mpo−/− mice after 24 hours of reperfusion. C6 deposition in Mpo−/− mice (C) was similar as compared with WT (B) controls. A: No C6 staining was detected between WT sham (not shown) and Mpo−/− sham-treated mice. Shown are representative microphotographs of all groups. Western blot results for C6 (sham in A; WT in B; KO in C) under reducing conditions are shown in the insets. Original magnifications, ×100.
Figure 10.
Similar common pathway activation between Mpo−/− (C) and WT (B) mice after 24 hours of reperfusion was confirmed by C9 immunostaining. WT sham (not shown) and Mpo−/− sham-treated mice (A) did not demonstrate any C9 staining. Original magnifications: ×100; ×200 (insets).
Discussion
The results provide evidence that a lack of MPO reduces renal function loss after I/R. By showing that MPO-deficient mice are partially protected from I/R-induced renal failure, the results demonstrate that this enzyme plays an important role in the pathogenesis of I/R-induced renal damage. MPO deficiency reduced PMN infiltration but failed to abrogate complement activation in reperfused murine kidneys.
MPO is a neutrophil-derived enzyme with the capacity to catalyze the formation of the proinflammatory oxidant HOCl and chlorinating species out of H2O2 and chloride ions.11 Apart from MPO’s contribution to innate immunity, there is in vitro evidence that MPO plays a role in apoptosis. Neutrophil-derived proteinase 3 and MPO mediate proapoptotic caspase-3 activation or induce direct HL-60 leukemia cell and endothelial cell apoptosis in vitro.34,35,36 Throughout the years, a massive body of literature has emerged describing the activation of apoptotic pathways in renal I/R.37,38 Furthermore, inhibition of apoptosis through administration of the anti-apoptotic agents such as IGF-1 or ZVAD-fmk (a broad caspase inhibitor) has been shown to preserve renal function after I/R.28 The hypothesis is tested that, in our model, a decrease in MPO-mediated apoptosis is one of the mechanisms through which Mpo−/− mice are protected from injury caused by renal I/R. Analyzing the overall levels of apoptosis, by detecting specific DNA fragmentation, it is shown that renal ischemia followed by 2 hours of reperfusion induces a marked increase in the level of apoptosis in both KO and WT animals. Moreover, the observed increase in apoptosis in Mpo−/− mice is similar to the rise of apoptosis in WT animals during this early phase of reperfusion. Similarly, studies by Vasilyev and colleagues19 could not show a significant contribution for MPO or its derived oxidants in the induction of apoptosis and necrosis in vivo. MPO rather adversely influenced organ function by the production of cytotoxic aldehydes19 or the oxidative inactivation of plasminogen activator inhibitor 1 (PAI-1).39 The present data indeed suggest that MPO has no significant in vivo role in the induction of renal cell death throughout the first moments of I/R but rather has a profound effect on organ function during late reperfusion, known as progression phase.
PMNs influence I/R-induced tissue damage in a multitude of organs by capillary plugging,9 induction of tubular leakage,40 release of oxygen-free radicals,41 and lysosomal enzyme activity.42,43,44,45 MPO specifically mediates neutrophil activation by binding to CD11b/CD18 (MAC1) integrins,32 as well as PMN adhesion via the αmβ2 integrin,33 thereby facilitating PMN extravasation. Inhibiting PMN extravasation abrogates renal I/R injury.14,46 The reduced levels of BUN, observed in this model in Mpo−/− mice, are accompanied by a decrease in PMN influx during late (24 hours) reperfusion. Our data are in line with the effect of MPO on PMNs and indicate that the absence of MPO prevents PMN activation and adhesion, thereby effectively reducing the amount of neutrophils invading the damaged tissue and preserving organ function.
A major role in the induction and continuation of local inflammation is played by the complement system. Complement proteins contribute to the development of I/R-induced organ injury.6,31,47 Predominantly the activation of the lectin30,31 and alternative pathway48 as well as the formation of the membrane attack complex (MAC)49,50 and small cationic proteins (C3a, C4a, and C5a), known as anaphylatoxins, have been shown to be involved in I/R-induced tissue injury.6,26 The deposition of early complement-activating proteins after 2 or 24 hours of reperfusion was similar between Mpo−/− and WT treated mice. Neutrophils produce several proteins, such as properdin and MPO, which have been shown to activate the complement cascade. Myeloperoxidase is reported to directly activate C5, generating a functional common pathway convertase capable of activating C6.20 We hypothesized that with the absence of MPO adequate means to locally activate common complement components in reperfused kidneys would be reduced. To elucidate common complement pathway activation, we analyzed C6 and C9 deposition in reperfused kidneys of Mpo−/− and WT controls after 24 hours of reperfusion. Similar to the activation of MBL and C3, no reduction in common complement pathway activity was shown in Mpo−/− mice. Our data suggest that complement activation initiated by the local presence of MPO is not of particular importance during renal I/R and consequently has no significant contribution to I/R-induced renal function loss. It has been described that several important proteins of the common complement cascade, such as C6 and C7,51 are directly produced by PMNs and released on their activation. This would mean that PMNs provide the necessary elements, ie, C6 and C7, to boost common pathway activation at sites of ongoing inflammation. This could imply a limited presence of these important common pathway components in case of a reduced PMN influx, as was observed in the reperfused kidneys of Mpo−/− mice. However, our data do not support that this quality attributed to the PMN has a detrimental role in our renal I/R model.
In conclusion, a reduced function loss in Mpo−/− mice as compared with WT controls was observed in a well established model of renal I/R. Apoptosis and activation of the early complement lectin and alternative pathway proteins, MBL-A, MBL-C, and C3, occurred similarly in Mpo−/− and WT mice during the first hours of reperfusion. After 24 hours of reperfusion, Mpo−/− mice exhibited preservation of renal function along with a strongly reduced number of neutrophils, present in the damaged renal tissue. The absence of MPO and the decrease in the number of neutrophils, however, did not correlate with a diminished activation of the common complement pathway in Mpo−/− mice. This observation might well illustrate that the contribution of MPO to renal organ damage after I/R is determined more by its influence on neutrophil extravasation and tissue infiltration than by its ability to mediate local complement activation in vivo. The results clarify important mechanisms by which PMNs and their derived activation products mediate I/R-induced renal injury on a local level.
Acknowledgments
We thank Jelly Nelissen and Isabelle Daisormont for their excellent technical assistance.
Footnotes
Address reprint requests to Dr. W.A. Buurman, Department of General Surgery, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands. E-mail: w.buurman@ah.unimaas.nl.
Supported by The Netherlands Organization for Health Research and Development (ZonMw grant 912-03-013 to W.A.B.), the Dutch Kidney Foundation (grant C01.1927 to D.H., J.W.C.T., and P.H.), and The Netherlands Organization for Scientific Research (NWO VIDI grant 917.66.341 to P.H.).
Disclosures: W.A.B. is shareholder of the company Hbt that provided some of the used antibodies. The antibodies, as used, are commercially available worldwide. The authors have no financial conflict of interest
Current address of P.H.: Department of Pathology and Laboratory Medicine, University of Groningen, Groningen, The Netherlands.
References
- Daemen MA, van de Ven MW, Heineman E, Buurman WA. Involvement of endogenous interleukin-10 and tumor necrosis factor-alpha in renal ischemia-reperfusion injury. Transplantation. 1999;67:792–800. doi: 10.1097/00007890-199903270-00003. [DOI] [PubMed] [Google Scholar]
- Daemen MA, van’t Veer C, Wolfs TG, Buurman WA. Ischemia/reperfusion-induced IFN-gamma up-regulation: involvement of IL-12 and IL-18. J Immunol. 1999;162:5506–5510. [PubMed] [Google Scholar]
- Thakur ML, Gottschalk A, Zaret BL. Imaging experimental myocardial infarction with indium-111-labeled autologous leukocytes: effects of infarct age and residual regional myocardial blood flow. Circulation. 1979;60:297–305. doi: 10.1161/01.cir.60.2.297. [DOI] [PubMed] [Google Scholar]
- Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363–1371. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- De Vries B, Matthijsen RA, Wolfs TG, Van Bijnen AA, Heeringa P, Buurman WA. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75:375–382. doi: 10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]
- Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein GW. Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed Proc. 1987;46:2397–2401. [PubMed] [Google Scholar]
- Kilgore KS, Lucchesi BR. Reperfusion injury after myocardial infarction: the role of free radicals and the inflammatory response. Clin Biochem. 1993;26:359–370. doi: 10.1016/0009-9120(93)90112-j. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Nagra RM, Becher B, Tourtellotte WW, Antel JP, Gold D, Paladino T, Smith RA, Nelson JR, Reynolds WF. Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J Neuroimmunol. 1997;78:97–107. doi: 10.1016/s0165-5728(97)00089-1. [DOI] [PubMed] [Google Scholar]
- Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol. 2001;159:2137–2145. doi: 10.1016/s0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malle E, Buch T, Grone HJ. Myeloperoxidase in kidney disease. Kidney Int. 2003;64:1956–1967. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- Vogt W. Complement activation by myeloperoxidase products released from stimulated human polymorphonuclear leukocytes. Immunobiology. 1996;195:334–346. doi: 10.1016/S0171-2985(96)80050-7. [DOI] [PubMed] [Google Scholar]
- Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huugen D, Xiao H, van Esch A, Falk RJ, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-alpha. Am J Pathol. 2005;167:47–58. doi: 10.1016/s0002-9440(10)62952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AF, Strath M, Sanderson CJ. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br J Haematol. 1984;57:489–494. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- Wolfs TG, de Vries B, Walter SJ, Peutz-Kootstra CJ, van Heurn LW, Oosterhof GO, Buurman WA. Apoptotic cell death is initiated during normothermic ischemia in human kidneys. Am J Transplant. 2005;5:68–75. doi: 10.1111/j.1600-6143.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- de Vries B, Kohl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA. Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol. 2003;170:3883–3889. doi: 10.4049/jimmunol.170.7.3883. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemen MA, van ’t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- Møller-Kristensen M, Wang W, Ruseva M, Thiel S, Nielsen S, Takahashi K, Shi L, Ezekowitz A, Jensenius JC, Gadjeva M. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol. 2005;61:426–434. doi: 10.1111/j.1365-3083.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- de Vries B, Walter SJ, Peutz-Kootstra CJ, Wolfs TG, van Heurn LW, Buurman WA. The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia-reperfusion injury. Am J Pathol. 2004;165:1677–1688. doi: 10.1016/S0002-9440(10)63424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brummer J, Rudolph V, Munzel T, Heitzer T, Meinertz T, Baldus S. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci USA. 2005;102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MW, Patarroyo M, Oberg F, Siegbahn A, Nilsson K. Myeloperoxidase mediates cell adhesion via the alpha M beta 2 integrin (Mac-1, CD11b/CD18). J Cell Sci. 1997;110:1133–1139. doi: 10.1242/jcs.110.9.1133. [DOI] [PubMed] [Google Scholar]
- Wagner BA, Buettner GR, Oberley LW, Darby CJ, Burns CP. Myeloperoxidase is involved in H2O2-induced apoptosis of HL-60 human leukemia cells. J Biol Chem. 2000;275:22461–22469. doi: 10.1074/jbc.M001434200. [DOI] [PubMed] [Google Scholar]
- Tsurubuchi T, Aratani Y, Maeda N, Koyama H. Retardation of early-onset PMA-induced apoptosis in mouse neutrophils deficient in myeloperoxidase. J Leukoc Biol. 2001;70:52–58. [PubMed] [Google Scholar]
- Myzak MC, Carr AC. Myeloperoxidase-dependent caspase-3 activation and apoptosis in HL-60 cells: protection by the antioxidants ascorbate and (dihydro)lipoic acid. Redox Rep. 2002;7:47–53. doi: 10.1179/135100002125000181. [DOI] [PubMed] [Google Scholar]
- Kaushal GP, Basnakian AG, Shah SV. Apoptotic pathways in ischemic acute renal failure. Kidney Int. 2004;66:500–506. doi: 10.1111/j.1523-1755.2004.761_6.x. [DOI] [PubMed] [Google Scholar]
- Dagher PC. Apoptosis in ischemic renal injury: roles of GTP depletion and p53. Kidney Int. 2004;66:506–509. doi: 10.1111/j.1523-1755.2004.761_7.x. [DOI] [PubMed] [Google Scholar]
- Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197:615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg PO, Kallskog TO. Neutrophil-mediated post-ischemic tubular leakage in the rat kidney. Kidney Int. 1989;36:555–561. doi: 10.1038/ki.1989.230. [DOI] [PubMed] [Google Scholar]
- Linas SL, Shanley PF, Whittenburg D, Berger E, Repine JE. Neutrophils accentuate ischemia-reperfusion injury in isolated perfused rat kidneys. Am J Physiol. 1988;255:F728–F735. doi: 10.1152/ajprenal.1988.255.4.F728. [DOI] [PubMed] [Google Scholar]
- Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. doi: 10.1038/ki.1997.200. [DOI] [PubMed] [Google Scholar]
- Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43:860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- Klausner JM, Paterson IS, Goldman G, Kobzik L, Rodzen C, Lawrence R, Valeri CR, Shepro D, Hechtman HB. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 1989;256:F794–F802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- Lin T, Zhou W, Farrar CA, Hargreaves RE, Sheerin NS, Sacks SH. Deficiency of C4 from donor or recipient mouse fails to prevent renal allograft rejection. Am J Pathol. 2006;168:1241–1248. doi: 10.2353/ajpath.2006.050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore KS, Ward PA, Warren JS. Neutrophil adhesion to human endothelial cells is induced by the membrane attack complex: the roles of P-selectin and platelet activating factor. Inflammation. 1998;22:583–598. doi: 10.1023/a:1022362413939. [DOI] [PubMed] [Google Scholar]
- Kilgore KS, Flory CM, Miller BF, Evans VM, Warren JS. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996;149:953–961. [PMC free article] [PubMed] [Google Scholar]
- Høgåsen AK, Wurzner R, Abrahamsen TG, Dierich MP. Human polymorphonuclear leukocytes store large amounts of terminal complement components C7 and C6, which may be released on stimulation. J Immunol. 1995;154:4734–4740. [PubMed] [Google Scholar]