Abstract
A multiprotein complex isolated from murine cells is identified as a counterpart of the yeast Mediator of transcriptional regulation on the basis of the following: homologs of two subunits of yeast Mediator, Srb7 and Med7, copurify with the complex; peptide sequencing reveals, in addition, homologs of the yeast Mediator subunits Rgr1 and Med6; as with yeast Mediator, the mouse complex binds to the RNA polymerase II C-terminal domain (CTD) and stimulates phosphorylation of the CTD by TFIIH. Peptide sequencing also identifies a component of mouse Mediator as a relative of Ring-3 protein, a mitogen-activated nuclear protein kinase, raising the possibility of Mediator as an end point of signal transduction pathways.
Keywords: MED genes/SRB genes/Ring-3/C-terminal domain/TFIIH kinase
Despite evidence for direct activator protein–basal transcription factor interaction (first reported in ref. 1), no response to activators can be detected in transcription reactions reconstituted with basal factors alone (for example, see ref. 2). Rather, additional proteins are required, which evidently play an intermediary role. Studies in Drosophila and human systems have identified TATA-binding protein-associated factors (TAFs) as such intermediaries (3, 4). Reconstitution of activated transcription with yeast proteins, however, revealed no requirement for TAFs and led to the isolation, instead, of a multiprotein Mediator complex (5). This apparent discrepancy between yeast and higher systems was underscored by the finding that destabilization or destruction of TAFs in yeast had no effect on activated transcription in vivo (6, 7). At a rare promoter where the elimination of TAFs did have an effect, their role was in recognition of an extended TATA element rather than in interaction with upstream regulatory sequences (8).
Several lines of evidence implicate Mediator in the response to upstream elements in yeast. The C-terminal domain (CTD) of RNA polymerase II is required for transcriptional activation; truncation of the CTD impairs activation, and suppressor mutations have been isolated in Mediator subunits termed Srbs (9). More direct evidence has come from mutations in the Mediator subunits Med2 and Med6, which diminish activation in vitro and in vivo (10, 11). Finally, mutations in other Mediator subunits, such as Sin4 and Rgr1, interfere with transcriptional repression (12–14).
The first indication of similar mechanisms in higher organisms, relating to the CTD, came from the expression of CTD-less RNA polymerase II in murine cells (15). Effects of almost all activator proteins tested were abolished. It has been shown further that human cells contain a homolog of the yeast Mediator subunit Srb7, which is enriched in a partially purified preparation of RNA polymerase II (16, 17). Database searches have revealed two additional human expressed sequence tags (ESTs) encoding homologs of the yeast Mediator subunits Med6 and Med7.
Do mammalian Srb7, Med6, and Med7 occur in a single, large complex? Do mammalian cells contain a physical and functional counterpart of the yeast Mediator of transcriptional regulation? Here we report on the existence of a mammalian protein complex containing multiple homologs of yeast Mediator components. Although isolated on the basis of sequence homology, the mammalian complex showed functional similarity to yeast Mediator as well.
Our findings also are pertinent to mechanisms of signal transduction in higher organisms. Two pathways of signal transduction culminating in effects on transcription have been described, involving nuclear translocation of transcription factors (reviewed in ref. 18) or of protein kinases (see, for example, refs. 19 and 20). The subunits of mouse Mediator described here include an apparent target of a cytosolic protein kinase, raising the possibility of Mediator as an end point of signal transduction pathways.
MATERIALS AND METHODS
Recombinant CTD.
His-tagged yeast CTD was expressed from a plasmid constructed by O. Gileadi (Weizmann Institute, Rehovot, Israel). For the expression of glutathione S-transferase (GST)-CTD, a HindIII fragment of YCpL14 (21) was filled in, cleaved with BamHI, and inserted into pGEX-2TK, which was cleaved previously with EcoRI, filled in, and cleaved with BamHI.
Antibodies and Immunoblot Analysis.
For the production of antibodies against mouse Med7 (GenBank accession no. AF031383), the DNA sequence from residues 4–131 was amplified by PCR from EST clone AA195756 with primers adding a 5′-NdeI site (5′-ATAGGATCCCATATGCCACAGCAAGTGAGTGCACTTCCACCACCTCC-3′) and a 3′-BamHI site (5′-CGCGGATCCGCTTGGTGGGGTCGGTATTCATTTATAAGCTGAGC-3′). The PCR product was inserted into the corresponding site of pET-16b (Novagen). The recombinant protein was expressed in Escherichia coli BL21(DE3)pLys, purified with the use of Ni2+-NTA agarose (Qiagen) under native conditions according to the manufacturer’s instructions, and used to immunize rabbits (Babco, Richmond, CA).
For the production of anti-hSrb7 antibodies, a 600-bp HindIII fragment of EST clone H08048 containing the full-length hSrb7 cDNA was inserted into the HindIII site of pUC1318, and a clone (D485) with hSrb7 and lacZ genes in opposite orientations was identified. A 600-bp BamHI fragment of D485 containing the full-length hSrb7 cDNA was inserted in the BamHI site of pET-16b, yielding a clone (D486) with an in-frame fusion of the hSrb7 cDNA with the His tag of the vector. His-tagged hSrb7 was expressed in BL21pLYSE, purified with the use of Ni2+-NTA agarose (Qiagen) according to the manufacturer’s instructions, and used to immunize rabbits (Babco). Unless otherwise noted, proteins were resolved in SDS-12% polyacrylamide gels and immunoblotted as described (5).
Mouse Mediator Purification.
Mouse Mediator was derived from nuclear extract, which was prepared as described (22) from about 4 kg of SpO/2 hybridoma cells. The nuclear extract was applied to a 9 × 30-cm column of BioRex 70 in buffer A(300) (25 mM Hepes, pH 7.9/1 mM DTT/1 mM EDTA/10% glycerol; mM potassium acetate in parenthesis). The column was washed with 2 vol of buffer A(600) and eluted with buffer A(1,200). Fractions containing mSrb7 and mMed7 were applied to a 4.5 × 25-cm column of hydroxyapatite in buffer B(10) (100 mM potassium acetate/1 mM DTT/0.1 mM CaCl2/10% glycerol/protease inhibitors (5); mM potassium phosphate, pH 7.7, in parenthesis). The column was washed with 2 vol of buffer B(350) and eluted with buffer B(500). Fractions containing mSrb7 and mMed7 were dialyzed against buffer C(100) (25 mM Tris acetate, pH 7.8/1 mM DTT/1 mM EDTA/10% glycerol/protease inhibitors; mM potassium acetate in parentheses) and applied to a MonoQ HR 10/10 column, developed with a linear gradient of potassium acetate (100–1,000 mM) in buffer C. Peak mSrb7 and mMed7 fractions (centered at about 800 mM potassium acetate) were made 1 M in ammonium sulfate and applied to a Phenyl Superose HR5/5 column in buffer D(1,000) (50 mM potassium phosphate, pH 7.0/1 mM DTT/10% glycerol/protease inhibitors; mM ammonium sulfate in parentheses), developed with a linear gradient of ammonium sulfate (1,000–0 mM) in buffer D. Peak mSrb7 and mMed7 fractions (centered at about 400 mM ammonium sulfate) were dialyzed against buffer C(100) and applied to a progel-TSK Heparin-5PW column in buffer C(100), developed with a linear gradient of potassium acetate (100–1,000 mM) in buffer C.
Protein Sequencing.
Ponceau S-stained protein bands (Fig. 3) were excised and digested with trypsin in situ (23). Peptides were fractionated by reversed-phase HPLC (24) with the use of a 0.8-mm Vydac C18 column. Selected peak fractions were analyzed by a combination of delayed extraction matrix-assisted laser-desorption/ionization reflectron time-of-flight mass spectrometry (REFLEX III, Bruker-Franzen; Bremen, Germany) and automated Edman sequencing (477A; Applied Biosystems) (25, 26). Peptide sequences were compared with entries in nonredundant protein and nucleotide (including dbEST) databases with the use of the National Center for Biotechnology Information Advanced blast program (27). Peptide monoisotopic masses were summed from the identified residues (including the presumed ones) with the use of procomp 1.2 software (obtained from P. C. Andrews, University of Michigan, Ann Arbor).
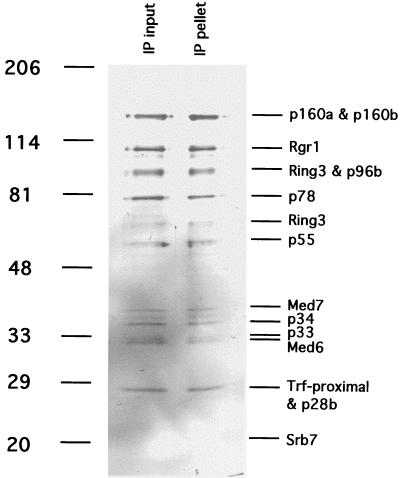
Figure 3.
Immunoprecipitation of mouse Mediator with antibodies against hSrb7. TSK-Heparin-5PW fraction 24 (50 μl) was immunoprecipitated with 100 μl of protein A-Sepharose-purified anti-hSrb7 antibodies crosslinked to 50 μl of Sepharose beads as described (5). After incubation for 4 h at 4°C in 25 mM Tris acetate, pH 7.8/200 mM potassium acetate/0.1 mM DTT/1 mM EDTA/10% glycerol/protease inhibitors, the beads were washed twice with the same buffer containing 500 mM potassium acetate/0.2% Nonidet P-40. Immunoprecipitated proteins (“IP pellet”) were eluted with 5 M urea twice for 10 min at room temperature, precipitated with trichloroacetic acid, analyzed alongside the starting fraction (“IP input,” 20 μl, precipitated with trichloroacetic acid) by electrophoresis in an 8–12% gradient SDS-polyacrylamide gel, and revealed by silver staining. Positions of bands due to size markers (kDa) are indicated at the left. Mediator subunits are identified at the right.
RESULTS
Homologs of Srb7 and Med7 Copurify with a Mouse Multiprotein Complex.
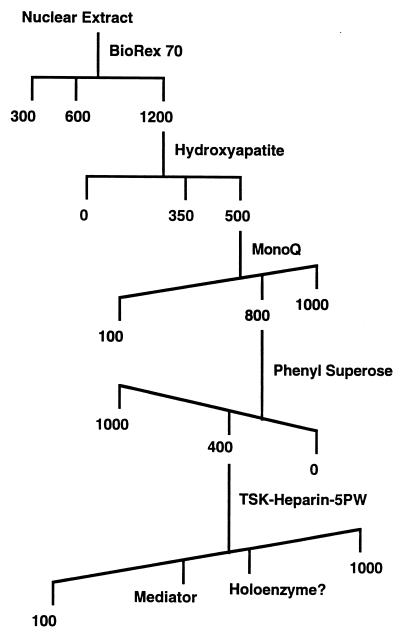
To investigate the occurrence of a mammalian protein complex containing multiple homologs of yeast Mediator proteins, a mouse hybridoma cell nuclear extract was fractionated by conventional means (Fig. 1), monitored by immunoblot analysis with antibodies against human Srb7 (hSrb7) and mouse Med7 (mMed7) proteins. Immunoreactive species with apparent molecular weights corresponding to mSrb7 and mMed7 were detected in the starting extract and copurified throughout the procedure. About half of these proteins eluted from BioRex 70 with 600 mM potassium acetate, and the remaining half was recovered in a 1,200 mM potassium acetate fraction. The 600 mM potassium acetate eluate proved refractory to further purification, possibly a result of damage caused by proteolysis, because the RNA polymerase II in this fraction was significantly degraded. The 1,200 mM fraction, on the other hand, could be enriched through four further chromatographic steps. The immunoreactive proteins eluted in two peaks in the final step, HPLC on TSK heparin (Fig. 2). The first peak coincided with a peak of several polypeptides revealed by SDS/PAGE.
Figure 1.
Mouse Mediator fractionation scheme. Numbers indicate concentrations (mM) of salts used for elution.
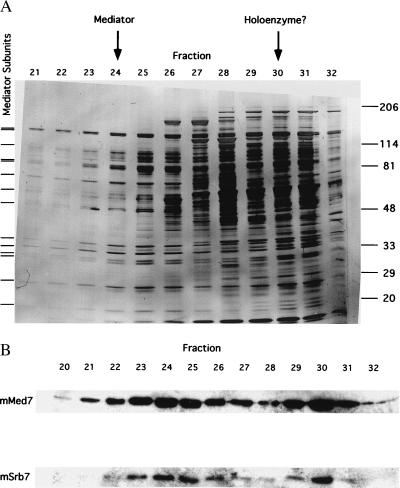
Figure 2.
Elution profiles from last step of mouse Mediator purification. (A) SDS/PAGE of TSK-Heparin-5PW fractions. Proteins were revealed by silver staining. Tick marks at left indicate positions of bands that coelute and coimmunoprecipitate (Fig. 3) and that are therefore attributed to Mediator subunits. Positions of bands due to size markers (kDa) are indicated at the right. Peak fractions of mSrb7 and mMed7 in the immunoblot (B Lower), attributed to Mediator and to possible RNA polymerase II–Mediator complex (“holoenzyme”), are indicated by arrows at the top. (B) Immunoblot of TSK-Heparin-5PW fractions with antibodies against hSrb7 and hMed7. Only the regions of the blots containing mSrb7 and mMed7 are shown.
Two observations identified the first peak from TSK heparin as a nearly homogeneous multiprotein complex. All polypeptides in the peak appeared to coelute, and all polypeptides were coimmunoprecipitated with anti-hSrb7 antibodies (Fig. 3). The second peak from TSK heparin evidently was impure, containing many more polypeptides. Reconstituted transcription assays with proteins from rat liver indicated the first peak was devoid of RNA polymerase II and general transcription factors, whereas the second peak contained polymerase, TFIIE, and TFIIH (data not shown).
Mouse Multiprotein Complex Contains Four Homologs of Yeast Mediator Subunits.
All bands resolved by SDS/PAGE from the first TSK heparin peak, except for those due to mSrb7 and mMed7, were subjected to mass spectrometric and limited sequence analysis (Table 1). The proteins were transferred to a nitrocellulose membrane and digested with trypsin. The resulting peptides were fractionated by microbore reversed-phase HPLC and analyzed by matrix-associated laser desorption/ionization time-of-flight mass spectrometry and limited Edman sequencing. Nine of the bands yielded sequences matching those of mouse or human ESTs. The band of apparent mass of 110 kDa (p110) exhibited homology to a Caenorhabditis elegans protein [basic local alignment search technique for protein sequences (BLASTP) value: 5.1e-35], which, in turn, shows significant homology to the yeast Mediator protein Rgr1 (BLASTP value: 8.4e-4) (Fig. 4A and B). Sequences from p96a showed homology with that of human Ring-3 protein (28), which, in turn, is homologous to a Drosophila developmental regulator, female sterile homeotic protein (29). Sequences from p66 identified it as a proteolytic digestion product of p96a. Sequences from p34 were homologous to that of a C. elegans protein of unknown function. Sequences from p32 identified it as a mouse version of the previously reported hMed6. The sequence of p28a showed homology with that of a Drosophila protein, TRF-proximal (Fig. 4C).
Table 1.
Mass spectrometric and limited sequence analysis results
| Subunit | Peptide sequence(s) | m/z | Database match(es) | [MH+] (Δppm) | Homolog(s) |
|---|---|---|---|---|---|
| p160a | — | — | — | ||
| p160b | — | — | — | ||
| p110 | LVTTDLPPQLANLTVANGR | 1,993.052 | EST AA562494 | 1,993.108 (28) | Yeast Rgr1 and C. elegans Rgr1 |
| LLQLEILVEDKETGDGR | 1,928.064 | EST AA562494 | 1,928.035 (15) | ||
| ALVHSMQIDFIHQLVQSR* | 2,122.097 | EST AA562494 | 2,122.123 (12) | ||
| p96a | YNPPDHEVVAMAR | 1,498.672 | EST AA145180 | 1,498.711 (26) | Human Ring-3 and Drosophila fsh |
| NSNPDEIEIDFETLKPSTLR | 2,318.128 | GenBank X97573 | 2,318.152 (32) | ||
| p96b | VASDTQFYPGLGLALAFQDGSVHMVHR | 2,916.306 | EST AA022304 | 2,916.446 (48) | — |
| p78 | TKPGSPHWQSK | 1,252.641 | EST HSDHEI067†/ | 1,252.644 (2) | — |
| TIGR hgi THC173176† | |||||
| IEDPQIQAHWSNINDVYESSVK | 2,572.194 | EST W84147 | 2,572.232 (14) | — | |
| QVQEVGLDGTETYLQPLSMSQNLAR | 2,777.376 | EST AA313890† | 2,777.378 (1) | — | |
| p66 | EYRDAQEFGADVR | 1,555.674 | EST AA145180/ | 1,555.714 (26) | A possible degradation |
| EST AA403891 | product of p96a | ||||
| p55 | — | — | — | ||
| p36 | — | — | Yeast Med7 | ||
| p34 | MGGVSGMAGLGSTR | 1,280.626 | EST D76720 MUS 69B04 | 1,280.610 (12) | C. elegans ZK546.13 gene product |
| RPYPTDLEMR | 1,277.630 | EST AA432609 | 1,277.632 (2) | ||
| ISASNAVCAPLTWVPGDPR | 2,059.030 | EST AA432609 | 2,059.045 (7) | ||
| P33 | RPYPTDLEMR | 1,277.618 | EST AA432609 | 1,277.632 (10) | A possible degradation product of p34 |
| P32 | RKEEPSSIFQR | 1,376.681 | EST AA608236/ | 1,376.730 (35) | Yeast Med6 |
| GENBANK U78082† | |||||
| VLTAVHGIQSAFDEAMSYCR* | 2,303.141 | EST AA608236 | 2,303.097 (19) | ||
| QSPAQVIPLADYYIIAGVIYQAPDLGSVINSR | 3,431.779 | EST AA613346† | 3,431.822 (12) | ||
| p28a | YQYCDFLVK | 1,283.614 | EST AA085480† | 1,283.614 (0) | Drosophila TRF |
| HDAVYGPADTMIQYMELFNK | 2,343.074 | EST AA085480† | 2,343.079 (2) | Proximal protein | |
| p28b | NFEAQLKPLVHLEK | 1,665.867 | W242958†/ | 1,665.934 (40) | — |
| AA461545†/ | |||||
| AA262939† | |||||
| p21 | — | — | Yeast Srb7 |
m/z, experimental monoisotopic mass. [MH+], calculated theoretical mass plus one proton. Δppm, difference between measured and theoretical mass, in parts per million.
Identified only by mass spectrometry.
Human ESTs.
Figure 4.
Sequence alignments. (A) Mouse and C. elegans Rgr1 homologs. Sequence analysis of the p110 component of mouse Mediator yielded two peptides (underlined) matching the sequence expected from mouse EST AA562494 translated in the +2 frame. The DNA sequence of EST AA562494 partially overlapped that of EST AA204093, and fusion of the two DNA sequences and translation generated the 233-residue mouse sequence shown. A blast search with this sequence revealed the homology with C. elegans Rgr1 shown. A third peptide from p110 was mapped to a region of the 233-residue mouse sequence (indicated by asterisks) on the basis of its experimental mass (m/z = 2122.097), which was in excellent agreement with the calculated monoisotopic mass of the expected peptide [(MH+) = 2122.123, Δ = 0.026 Da (12 ppm)]. (B) Yeast Rgr1 and C. elegans Rgr1 homolog. (C) Human homolog of the p28a component of mouse Mediator and Drosophila Trf-proximal. Peptide sequences obtained from p28a are underlined. A difference between human and mouse sequences is indicated by an asterisk.
Mouse Multiprotein Complex Binds the RNA Polymerase II CTD and Stimulates CTD Phosphorylation.
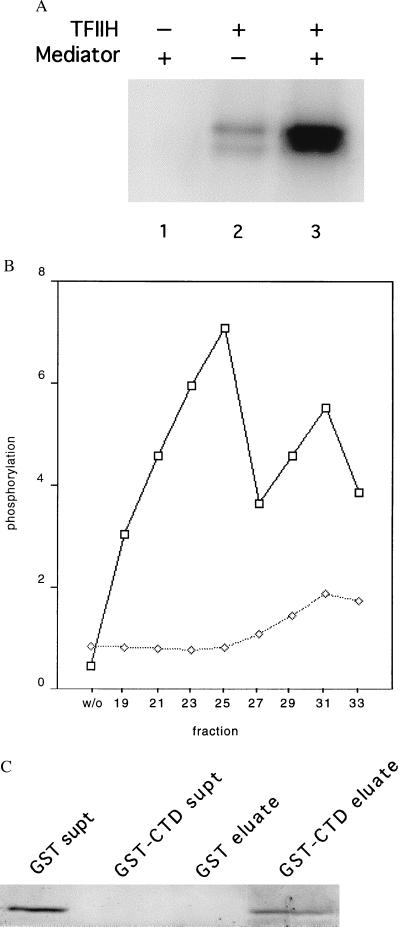
Purified yeast Mediator exhibits three functional activities: stimulation of basal transcription, support of activated transcription, and stimulation of CTD phosphorylation by TFIIH. Yeast Mediator has further been shown to bind tightly and specifically to the CTD. Assays performed with purified RNA polymerase II and general transcription factors from rat liver so far have failed to reveal effects of the multiprotein complex from the first TSK heparin peak on either basal or activated reactions. Stimulation of CTD phosphorylation, however, was readily observed. In reactions performed with recombinant yeast CTD and rat TFIIH, the increase in phosphorylation in the presence of a TSK heparin fraction was 9.5-fold (Fig. 5A). The stimulatory activity evidently resided in the mSrb7/mMed7 multiprotein complex, since the profile of activity across the TSK heparin column corresponded with that of the immunoreactive polypeptides (Fig. 5B, upper curve). Some activity could be detected in the second TSK heparin peak even without the addition of TFIIH, presumably reflecting the presence of TFIIH in that peak, as mentioned above (Fig. 5B, lower curve).
Figure 5.
Physical and functional interaction of mouse Mediator with the RNA polymerase II CTD. (A) Stimulation by mouse Mediator of CTD phosphorylation by TFIIH. Recombinant His-tagged yeast CTD (50 ng) was incubated with rat TFIIH (15 ng) and mouse Mediator (TSK-Heparin-5PW fraction 25, 100 ng) in 15 μl of 20 mM Hepes, pH 7.6/110 mM potassium acetate/5 μM (0.3 μCi) [γ-32P]ATP/7.5 mM magnesium acetate/2 mM DTT for 30 min at 23°C, followed by electrophoresis in an SDS-polyacrylamide gel and autoradiography. (B) Profile of stimulatory activity shown in A across the TSK-Heparin-5PW column (1 μl of each fraction, corresponding to 100 ng of fraction 25, or control without fraction, designated “w/o”), measured in the presence (upper curve) or absence (lower curve) of added TFIIH. Intensities of bands determined with a PhosphorImager are plotted in arbitrary units on the abscissa. (C) Binding of mouse Mediator to GST-CTD. Glutathione-agarose (30 μl of a 50% slurry, Sigma) was incubated with GST (50 μg) or GST-CTD (50 μg) for 1 h at 4°C with gentle agitation. The beads were washed three times with buffer E(600) (20 mM Tris⋅Cl, pH 7.9/1 mM MgCl2/10 μM ZnCl2/0.2 mM EDTA/10% glycerol/protease inhibitors; mM potassium acetate in parenthesis) containing 0.02% Nonidet P-40, and twice in buffer E(200) containing 0.01% Nonidet P-40. The beads then were incubated with Mediator (TSK-Heparin-5PW fraction 25, 1.5 μg) in 100 μl of buffer E(200) containing 0.01% Nonidet P-40 for 2 h at 4°C with gentle agitation, followed by three washes with buffer E(200) containing 0.1% Nonidet P-40 and elution with 35 μl of 10 mM reduced glutathione in 50 mM Tris acetate, pH 7.8, for 10 min at 4°C with gentle agitation. The supernatants from the initial binding of Mediator, concentrated by trichloroacetic acid precipitation (“GST supt,” “GST-CTD supt”), and the entire eluates were analyzed by SDS/PAGE and immunoblotting with anti-mMed7 antibodies.
Binding of the multiprotein complex from the first TSK heparin peak to the yeast CTD was demonstrated with the use of a GST-CTD fusion protein immobilized on glutathione-agarose. The complex was entirely bound by the resin and could be eluted completely with glutathione, as shown by immunoblot analysis with anti-mMed7 antibodies (Fig. 5C). No binding was detected to a control resin formed with GST alone.
DISCUSSION
Four lines of evidence identify the mouse multiprotein complex isolated here as a mammalian counterpart of the yeast Mediator of transcriptional regulation. First, homologs of the yeast Mediator subunits Srb7 and Med7 copurify with the multiprotein complex. Occurrence of the entire set of proteins in a discrete entity was shown by coimmunoprecipitation with anti-hSrb7 antibodies. Second, peptide sequence determination revealed homologs of the yeast Mediator subunits Rgr1 and Med6 in the multiprotein complex as well. Third, the complex binds specifically to the RNA polymerase II CTD, as does yeast Mediator. Finally, the complex stimulates TFIIH phosphorylation of the CTD, a key functional characteristic of yeast Mediator.
The differences between yeast and mammalian Mediators also may be significant. Only four of nine mouse Mediator subunits for which peptide sequence information is available have homologs in yeast. Mouse Mediator appears to lack all members of a set of proteins, Sin4, Med2, Pgd1, and Gal11, which form a discrete module of yeast Mediator (“Sin4 module”) (11, 30). Whereas databases contain multiple entries of ESTs encoding the four mouse homologs of yeast Mediator proteins so far identified, no ESTs encoding peptides homologous to members of the Sin4 module could be found. Mouse Mediator also evidently lacks a point of attachment of the Sin4 module, which, in the yeast complex, is through the C-terminal region of Rgr1 (30). Although a full-length mammalian Rgr1 sequence has not yet been determined, the high degree of similarity of the N-terminal region between yRgr1 and cRgr1 and lack of other homology in the sequence currently available (Fig. 4B) suggest that homology between the two proteins is restricted to the N-terminal region.
The discovery of a Ring-3-like protein as a subunit of Mediator points to a role of the mouse complex in signal transduction as well as in transcriptional regulation. Genetic studies have suggested that a Drosophila homolog of Ring-3, the female sterile homeotic (fsh) gene, is a transacting effector of trithorax, another homeotic gene, whose sequence is indicative of a role as a transcription factor. A human homolog of trithorax, ALL-1 (also known as MLL, HRX, and HTRX-1) (31–35), is altered by reciprocal chromosomal translocations in certain leukemias. Human Ring-3 has been identified as a nuclear kinase, which is activated by phosphorylation. The kinase activity is elevated markedly in acute and chronic lymphocytic leukemias. The genetic interaction between Drosophila trithorax and fsh prompted the proposal that ALL-1 is a target of the human Ring-3 kinase (36). The occurrence of a Ring-3-like protein in mouse Mediator raises the possibility that the Ring-3 target is a component of the RNA polymerase II transcription machinery. Regardless of the Ring-3 target, the involvement of Mediator in signal transduction pathway(s) affecting transcription calls for further investigation.
Besides Ring-3, a 90-kDa, Ring-3-like kinase has been isolated from Hela nuclear extracts that is activated in response to mitogens by phosphorylation, apparently as a result of nuclear translocation of a cytosolic protein kinase (36). Although others originally identified the protein as Ring-3, we find a perfect match of published peptide sequences from the 90-kDa protein with the predicted product of the human orfx gene, a Ring-3-like protein.
In addition to the regulation of human Ring-3 by phosphorylation, the occurrence of multiple Ring-3-like proteins encoded by human ESTs raises the possibility of modulation of mediator function by variation of the Ring-3 subunit. Multiple Ring-3-like proteins also have been identified in the mouse, and the peptide sequences we obtained for p96a are insufficient to correlate it more precisely with a particular human protein. Studies of the mammalian Ring-3 family have revealed both cell-type and functional specificity. A human family member, BRDT, is expressed specifically in testis (37). Expression of a rat Ring-3-like gene, Mud6, is induced by programmed cell death in cultured neuronal cells and developing sympathetic neurons (38). The Drosophila fsh gene also displays specificity, functioning in embryogenesis and exhibiting a maternal effect (29).
The possible occurrence of multiple forms of Mediator also could account for our failure to observe activity in transcription in vitro. Our trials to date have been limited to the activator Gal4-VP16 and the adenoviral major late promoter, which may not respond to the Mediator we have isolated from mouse hybridoma cells. Other possible reasons for inactivity of the purified Mediator include the loss of an essential component(s) during isolation, or a requirement for modification, such as phosphorylation of the Ring-3-like protein (36).
Acknowledgments
We thank Protein Design Labs, Inc., for a generous gift of mouse hybridoma cells, Opher Gileadi for an expression plasmid for His-tagged yeast CTD, Robert M. Brazas for testing anti-hSrb7 antibodies, Averell Gnatt for help with preparation of anti-Srb7 Sepharose, and Mary Lui and Lynne Lacomis for expert assistance with protein structural analysis. Y.W.J. is a Damon Runyon postdoctoral fellow, and P.V. is a Human Frontiers Science Program postdoctoral fellow. J.W.C. is an associate investigator of the Howard Hughes Medical Institute. This research was supported by National Institutes of Health Grant GM36659 to R.D.K., National Institutes of Health Grant GM41628 to R.C.C., National Science Foundation Grant DBI-940123 to P.T., and National Cancer Institute Grant 5 P30 CA08748 to the Sloan–Kettering Structural Chemistry Laboratory.
ABBREVIATIONS
- CTD
C-terminal domain
- EST
expressed sequence tag
- GST
glutathione S-transferase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF031383).
References
- 1.Stringer K F, Ingles C J, Greenblatt J. Nature (London) 1990;345:783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- 2.Flanagan P M, Kelleher R J, III, Sayre M H, Tschochner H, Kornberg R D. Nature (London) 1991;350:436–438. doi: 10.1038/350436a0. [DOI] [PubMed] [Google Scholar]
- 3.Pugh B F, Tjian R. Cell. 1990;61:1187–1197. doi: 10.1016/0092-8674(90)90683-6. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q, Liberman P M, Boyer T G, Berk A J. Genes Dev. 1992;6:1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y J, Bjorklund S, Li Y, Sayre M H, Kornberg R D. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 6.Walker S S, Reese J C, Apone L M, Green M R. Nature (London) 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 7.Moqtaderi Z, Bai Y, Poon D, Weil P A, Struhl K. Nature (London) 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 8.Shen W-C, Green M R. Cell. 1997;90:615–624. doi: 10.1016/s0092-8674(00)80523-1. [DOI] [PubMed] [Google Scholar]
- 9.Koleske A J, Buratowski S, Nonet M, Young R A. Cell. 1992;69:883–894. doi: 10.1016/0092-8674(92)90298-q. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y C, Min S, Gim B S, Kim Y J. Mol Cell Biol. 1997;17:4622–4632. doi: 10.1128/mcb.17.8.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers L C, Gustafsson C M, Bushnell D A, Lui M, Erdjument-Gromage H, Tempst P, Kornberg R D. Genes Dev. 1998;12:45–54. doi: 10.1101/gad.12.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Y, Stillman D J. Mol Cell Biol. 1992;12:4503–4514. doi: 10.1128/mcb.12.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai A, Shimizu Y, Kondou S, Chibazakura T, Hishinuma F. Mol Cell Biol. 1990;10:4130–4138. doi: 10.1128/mcb.10.8.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covitz P A, Song W, Mitchell A P. Genetics. 1994;138:577–586. doi: 10.1093/genetics/138.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber H-P, Hagmann M, Seigal K, Georgiev O, West M A L, Litingtung Y, Schaffner W, Corden J L. Nature (London) 1995;374:660–662. doi: 10.1038/374660a0. [DOI] [PubMed] [Google Scholar]
- 16.Chao D M, Gadbois E L, Murray P J, Anderson S F, Sonu M S, Parvin J D, Young R A. Nature (London) 1996;380:82–85. doi: 10.1038/380082a0. [DOI] [PubMed] [Google Scholar]
- 17.Maldonado E, Shiekhattar R, Sheldon M, Cho H, Drapkin R, Rickert P, Lees E, Anderson C W, Linn S, Reinberg D. Nature (London) 1996;381:86–89. doi: 10.1038/381086a0. [DOI] [PubMed] [Google Scholar]
- 18.Whiteside S T, Goodbourn S. J Cell Sci. 1993;104:949–955. doi: 10.1242/jcs.104.4.949. [DOI] [PubMed] [Google Scholar]
- 19.Leach K L, Powers E A, Ruff V A, Jaken S, Kaufman S. J Cell Biol. 1989;109:685–695. doi: 10.1083/jcb.109.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigg E A, Hilz H, Eppenberger H M, Dutly F. EMBO J. 1985;4:2801–2806. doi: 10.1002/j.1460-2075.1985.tb04006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Kornberg R D. Proc Natl Acad Sci USA. 1994;91:2362–2366. doi: 10.1073/pnas.91.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dignam J D, Lebovitz R M, Roeder R G. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lui M, Tempst P, Erdjument-Bromage H. Anal Biochem. 1996;241:156–166. doi: 10.1006/abio.1996.0393. [DOI] [PubMed] [Google Scholar]
- 24.Elicone C, Lui M, Geromanos S, Erdjument-Bromage H, Tempst P. J Chromatogr. 1994;676:121–137. doi: 10.1016/0021-9673(94)00089-1. [DOI] [PubMed] [Google Scholar]
- 25.Tempst P, Geromanos S, Elicone C, Erdjument-Bromage H. Methods Companion Methods Enzymol. 1994;6:248–261. [Google Scholar]
- 26.Erdjument-Bromage H, Lui M, Sabatini D M, Snyder S H, Tempst P. Protein Sci. 1994;3:2435–2446. doi: 10.1002/pro.5560031227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Beck S, Hanson I, Kelly A, Pappin D J C, Trowsdale J. DNA Seq. 1992;2:203–210. doi: 10.3109/10425179209020804. [DOI] [PubMed] [Google Scholar]
- 29.Haynes S R, Mozer B A, Bhatia-Dey N, Dawid I B. Dev Biol. 1989;134:246–257. doi: 10.1016/0012-1606(89)90094-8. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Bjorklund S, Jiang Y W, Kim Y-J, Lane W S, Stillman D J, Kornberg R D. Proc Natl Acad Sci USA. 1995;92:10864–10868. doi: 10.1073/pnas.92.24.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cimino G, Moir D T, Canaani O, K, W, Crist W M, Katsav S, Cannizzaro L, Lange B, Nowell P C, Croce C M, Canaani E. Cancer Res. 1991;51:6712–6714. [PubMed] [Google Scholar]
- 32.Djabali M, Selleri L, Parry P, Bower M, Young B D, Evans G A. Nat Genet. 1992;2:113–118. doi: 10.1038/ng1092-113. [DOI] [PubMed] [Google Scholar]
- 33.Gu Y, Nakamura H, Alder H, Prasad R, Canaani O, Cimino G, Croce C M, Canaani E. Cell. 1992;71:701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- 34.Tkachuk D C, Kohler S, Cleary M L. Cell. 1992;71:691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 35.Ford A M, Ridge S A, Cabrera M E, Mahmoud H, Steel C M, Chan L C, Greaves M. Nature (London) 1993;363:358–360. doi: 10.1038/363358a0. [DOI] [PubMed] [Google Scholar]
- 36.Denis G V, Green M R. Genes Dev. 1996;10:261–271. doi: 10.1101/gad.10.3.261. [DOI] [PubMed] [Google Scholar]
- 37.Jones M H, Numata M, Shimane M. Genomics. 1997;45:529–534. doi: 10.1006/geno.1997.5000. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, DiBenedetto A J, Pittman R N. Dev Biol. 1997;188:322–336. doi: 10.1006/dbio.1997.8655. [DOI] [PubMed] [Google Scholar]