Abstract
Synergy between Toll-like receptor (TLR) and adenosine A2A receptor (A2AR) signaling switches macrophages from production of inflammatory cytokines such as tumor necrosis factor-α to production of the angiogenic growth factor vascular endothelial growth factor (VEGF). We show in this study that this switch critically requires signaling through MyD88, IRAK4, and TRAF6. Macrophages from mice lacking MyD88 (MyD88−/−) or IRAK4 (IRAK4−/−) lacked responsiveness to TLR agonists and did not respond to A2AR agonists by expressing VEGF. Suppression of TRAF6 expression with siRNA in RAW264.7 macrophages also blocked their response to TLR and A2AR agonists. Excisional skin wounds in MyD88−/− mice healed at a markedly slower rate than wounds in wild-type MyD88+/+ mice, showing delayed contraction, decreased and delayed granulation tissue formation, and reduced new blood vessel density. Although macrophages accumulated to higher levels in MyD88−/− wounds than in controls, expression of VEGF and HIF1-α mRNAs was elevated in MyD88+/+ wounds. CGS21680, an A2AR agonist, promoted repair in MyD88+/+ wounds and stimulated angiogenesis but had no significant effect on healing of MyD88−/− wounds. These results suggest that the synergistic interaction between TLR and A2AR signaling observed in vitro that switches macrophages from an inflammatory to an angiogenic phenotype also plays a role in wound healing in vivo.
Macrophages play a key role in host defense by recognizing and destroying foreign organisms, debriding dead and damaged tissue components, including fibrin and apoptotic cells, and producing cytokines, growth factors, and angiogenic factors that regulate inflammation, angiogenesis, and granulation tissue formation.1,2,3,4,5 Macrophages express a series of cell surface transmembrane receptors that mediate the recognition of a variety of components of foreign organisms.6,7,8,9,10,11,12 These include type A scavenger receptors, CD36, CD68, CD14, CD11b/CD18, dectin, and several members of the Toll-like receptor (TLR) family.4,8,9,13,14,15 TLRs are pattern recognition receptors that possess an extracellular domain with leucine-rich repeats that participate in ligand recognition, a trans-membrane domain, and a cytoplasmic domain termed the Toll-IL-1 receptor (TIR) domain that has sequence homology to the interleukin (IL)-1R cytoplasmic tail.16 TLRs are major initiators of innate immune responses, and agonists of TLRs induce the expression by macrophages of several cytokines, such as tumor necrosis factor (TNF)-α and IL-12, that participate in inflammation and wound healing. TLR signaling is transmitted through cytoplasmic adaptor proteins such as MyD88, TIRAP (MAL), TRIF, and TRAM, which associate with cytoplasmic TIR domains of TLRs.17,18 MyD88 is a critical signal adaptor for TLRs 2, 4, 5, 7, 8, 9, and 11, whereas TLR3 signals in a MyD88-independent manner through the TRIF adapter pathway. MyD88 is also a critical signaling mediator for IL-1 and IL-18 signaling.19 MyD88 signaling involves recruitment and activation of IRAK4, IRAK1, and TRAF6, resulting ultimately in the activation of the NF-κB, AP1, and MAP kinase pathways.20
Adenosine is a metabolite produced by the breakdown of ATP under conditions of cell stress, such as ischemia, hypoxia, and inflammation.21,22 Adenosine signals through a group of four G-protein-coupled seven-transmembrane receptors (A1R, A2AR, A2BR, and A3R) and has numerous effects on both the microvasculature as well as on inflammatory cells.23,24,25 The effects of adenosine are generally anti-inflammatory, and adenosine strongly down-regulates the expression of cytokines such as TNF-α, IL-12, and MIP1-α by macrophages.26,27,28,29 We have shown that in the presence of TLR agonists such as endotoxin (lipopolysaccharide, LPS), adenosine strongly and synergistically up-regulates the expression of the angiogenic growth factor vascular endothelial growth factor (VEGF) by macrophages, thus acting as an angiogenic switch.30 This effect of adenosine is independent of hypoxia and is mediated through the A2AR.28 Macrophages from mice lacking A2ARs express inflammatory genes such as TNF-α and IL-12 in response to TLR agonists but fail to undergo the angiogenic switch in response to A2AR agonists. The mechanism by which TLR agonists synergize with A2AR agonists to switch macrophages from an inflammatory to an angiogenic phenotype is not yet clear. We have shown that the synergistic up-regulation of VEGF by LPS (a TLR4 agonist) and A2AR agonists is mediated by the transcriptional induction of VEGF expression through the up-regulated expression of HIF1-α. LPS (a TLR4 agonist) transcriptionally up-regulates HIF1-α expression in an A2AR-independent manner, and A2AR agonists strongly stabilize the LPS-induced HIF1-α mRNA.31 Adenosine A2AR agonists have been shown to promote wound healing and granulation tissue formation in vivo.32,33 Wound healing in mice lacking the A2AR (A2AR knockout mice) has been shown to be markedly impaired, with decreased and delayed formation of granulation tissue.33,34
In this study, we have examined the role of MyD88-dependent signaling on the angiogenic switch in macrophages both in vitro and in vivo. MyD88 is a critical downstream mediator of signaling through most TLRs, including TLRs 2, 4, 7, and 9, which mediate the angiogenic switch. MyD88 associates with the cytoplasmic TIR domain of TLRs and mediates signaling through recruitment of a signaling complex that includes IRAK1, IRAK4, and TRAF6.17,20,35,36,37,38 Using macrophages from knockout mice and siRNA to suppress specific gene expression in macrophages, we find that MyD88 signaling is critical for the angiogenic switch and that IRAK4 and TRAF6, both downstream mediators of MyD88 signaling, are also critically required. In addition, we have studied dermal wound healing in vivo and show that MyD88-deficient mice have a severely impaired wound healing phenotype characterized by delayed granulation tissue formation and reduced blood vessel formation. To determine whether A2AR signaling plays a role in this phenotype, we have tested the effects of CGS21680, a specific A2AR agonist, on wound healing in MyD88 knockout mice, to determine whether the previously reported A2AR-mediated regulation of wound healing requires the presence of intact MyD88 signaling.32,33,39,40,41 Our results indicate that the promotional effects of CGS21680 observed in wild-type mice are lost in MyD88 knockout mice. This suggests that MyD88-dependent signaling is required for the promotional effects of this A2AR agonist and that the synergistic pathway involving TLR and A2AR signaling that we have defined in vitro in macrophages may also play a role in the regulation of wound healing in vivo.
Materials and Methods
Chemicals and Reagents
5′-N-ethylcarboxamidoadenosine (NECA) (an agonist that acts as a ligand for both A2ARs and A2BRs) and 2-[p-(2-carboxyethyl)-phenylethyl amino]-5′-N-ethyl carboxamidoadenosine (CGS21680) (a specific A2AR agonist) were purchased from Sigma Chemical Co. (St. Louis, MO). ZM241385 (a specific antagonist of A2ARs) was purchased from Tocris-Cookson (Bristol, UK). Resiquimod (R848) (a TLR7 agonist) was purchased from Invivogen (San Diego, CA). The TLR4 agonist LPS (phenol-purified Escherichia coli serotype 055:B5 free of TLR2 agonists) was a gift of Dr. Stefanie Vogel (University of Maryland, College Park, MD). An affinity-purified goat anti-CD31 polyclonal antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). A rat IgG2b anti-mouse macrophage monoclonal antibody (F4/80) was affinity purified from hybridoma supernatant using a Protein G agarose kit (KPL, Gaithersburg, MD). Antibodies for secondary immunohistochemical staining were purchased from Santa Cruz Biotechnology Inc.
Animals
C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the New Jersey Medical School animal facility. Mice on the C57BL/6J background with a specific deletion of the MyD88 gene (MyD88−/− mice) were kindly provided by Dr. Shizuo Akira (Osaka University, Osaka, Japan).42,43 Heterozygous mice (MyD88+/−) were bred to provide MyD88−/− and MyD88+/+ mice as littermate controls. IRAK4−/− mice on the C57BL/6J background were kindly provided by Dr. Wen Chen Ye at the University of Toronto, Toronto, Canada, and were also bred to provide IRAK4−/− and IRAK4+/+ mice as littermate controls.36,44 MyD88 and IRAK4 colonies were maintained in a barrier facility under specific pathogen-free conditions. All animal procedures were reviewed and approved by the New Jersey Medical School Institutional Animal Care and Use Committee.
Preparation of Macrophages
Mouse peritoneal macrophages were harvested as previously described with some modifications.30 Mice (7 to 8 weeks of age) were injected intraperitoneally with 2.5 ml of thioglycollate broth, and 4 days later the peritoneal macrophages were harvested and cultured as a monolayer in RPMI 1640 medium (Cellgro; Mediatech Inc., Herndon, VA) supplemented with 10% fetal bovine serum (Gemini Bio-Products, Calabasas, CA), 2 mmol/L l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Irvine Scientific, Santa Ana, CA). The cultures were found to be >98% pure as assessed by nonspecific esterase staining and staining with the macrophage-specific F4/80 monoclonal antibody.
Cell Culture
RAW 264.7 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gemini Bio-Products), 2 mmol/L l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Irvine Scientific). The cells were grown at 37°C in a humidified incubator in 5% CO2 and 95% air.
Preparation of pSilencer Plasmids with siRNA Constructs
Specific hairpin siRNA oligonucleotide constructs for murine TRAF6 silencing were designed using the Ambion siRNA design algorithm, synthesized in the New Jersey Medical School Biotechnology Central facility, and cloned into the pSilencer3.1-H1neo expression vector (Ambion Inc., Austin, TX), following the manufacturer’s protocol. siRNA constructs were designed targeting both the 5′ end and the 3′ of the gene. As controls, nonspecific siRNA with the same nucleotide composition as the specific inserts, but lacking significant homology with any sequence in the murine genome database, were also prepared. The specific complementary siRNA constructs for TRAF6 are shown in Tables 1 and 2. The plasmids were transfected into XL10-Gold ultracompetent cells (Stratagene, La Jolla, CA), and plasmid DNA was isolated using the MAXIPREP GFII endo-free kit (Sigma). Plasmids were sequenced to ensure the fidelity of the cloned inserts.
Table 1.
Sequences of Specific and Scrambled siRNA Constructs for Silencing of TRAF6
| 5′ end (specific) | |
| Upper | 5′-GATCCGCAGAGCTACTATGAGTCTCTTCAAGAGAGAGACTCATAGTAGCTCTGTTTTTTGGAAA-3′ |
| Lower | 5′-AGCTTTTCCAAAAAACAGAGCTACTATGAGTCTCTCTCTTGAAGAGACTCATAGTAGCTCTGCG-3′ |
| 3′ end (specific) | |
| Upper | 5′-GATCCGTACTGATGCGGGGGTGTATTCAAGAGATACACCCCCGCATCAGTACTTTTTTGGAAA-3′ |
| Lower | 5′-AGCTTTTCCAAAAAAGTACTGATGCGGGGGTGTATCTCTTGAATACACCCCCGCATCAGTACG-3′ |
| 5′ end (scrambled) | |
| Upper | 5′-GATCCGCAGTCTAGCGTACATCATTTCAAGAGAATGATGTACGCTAGACTGCTTTTTTGGAAA-3′ |
| Lower | 5′-AGCTTTTCCAAAAAAGTATGAGGGCGACTGTTGGTCTCTTGAACCAACAGTCGCCCTCATACG-3′ |
| 3′ end (scrambled) | |
| Upper | 5′-GATCCGTATGAGGGCGACTGTTGGTTCAAGAGACCAACAGTCGCCCTCATACTTTTTTGGAAA-3′ |
| Lower | 5′-AGCTTTTCCAAAAAAGTATGAGGGCGACTGTTGGTCTCTTGAACCAACAGTCGCCCTCATACG-3′ |
Table 2.
Primer and Probe Sequences for Quantitative RT-PCR Analysis of TRAF6 mRNA
| Forward | 5′-TGGCCACAGGTTCTGCAAA-3′ |
| Reverse | 5′-TTTCCAGCAGTATTTCATTGTCAAC-3′ |
| Probe | 5′-FAM-ATCAATCCATAAGGGATGCAGGGC AC–BHQ-3′ |
Preparation of RAW264.7 Cell-Stable Transfectants
RAW264.7 cells were transfected with siRNA-containing plasmids using Superfect transfection reagent (Qiagen Inc., Valencia, CA), following the manufacturer’s protocol. Briefly, cells were plated 1 day before transfection in 60-mm culture dishes. Cells were then incubated with Superfect transfection complex with siRNA plasmids in growth medium for 3 hours. The medium was then removed and replaced with fresh medium supplemented with 0.8% (w/v) G418. This selection medium was replaced every 2 days. G418-resistant colonies were selected after ∼2 weeks of growth. Six single clones of each siRNA transfection were selected and grown in T25 flasks. G418 was maintained in the medium for the growth of the clones.
Isolation of Total Cellular RNA
Total cellular RNA was isolated from macrophages or RAW264.7 cells using Trizol reagent (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s protocol for isolation of total RNA from animal cells. RNA was quantified using SYBR Green II (Sigma) according to the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (PCR) was performed using TaqMan technology and an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA).
Isolation of Total RNA from Wound Tissues
Wounds with ∼2 mm of surrounding skin were excised at the indicated time points and flash-frozen in liquid nitrogen. The wound tissues were dissected away from the surrounding skin and rapidly homogenized in Trizol reagent. RNA was then isolated and quantitated as described above. Real-time quantitative PCR was performed using TaqMan technology and ABI 7500 real-time PCR system as described below.
Real-Time PCR
Complimentary DNA (cDNA) was synthesized from total RNA using TaqMan reverse transcription reagents (Applied Biosystems) following the manufacturer’s instructions. Real-time polymerase chain reaction was performed in an ABI Prism 7500 sequence detector using 1/10th volume of each cDNA reaction and TaqMan Universal PCR master mix. The TaqMan gene expression assay for cyclophilin D (Mm00835365_g1) was purchased from Applied Biosystems. Primers and probes for mouse TRAF6 mRNA were designed using Applied Biosystems’ Primer Express 2.0 software and synthesized at the Molecular Resource Facility at NJMS-UMDNJ (Newark, NJ). 5′ Ends of the probes were labeled with the fluorescent dye 6-carboxy fluorescein (FAM) and 3′ ends were coupled to the quencher molecule Black Hole Quencher dye-1 (BHQ-1). Sequences of primers and probes are presented in Tables 1 and 2. Primers and probes for murine VEGF-A and HIF1-α were identical to those we have described previously.45 The real-time PCR reactions were performed using the manufacturer’s protocol for absolute quantification. After initial denaturation at 95°C for 10 minutes, reactions were subjected to 40 cycles of polymerase chain reaction, each cycle consisting of 15 seconds at 95°C, followed by 60 seconds at 60°C. For each sample, gene expression levels were normalized to that of endogenous cyclophilin D.
Western Blot Analyses
RAW264.7 cells were plated in 100-mm dishes. After overnight incubation, the medium was changed to RPMI 1640 with 1% fetal bovine serum, and the cells were incubated alone, stimulated with NECA (1 μmol/L), LPS (100 ng/ml), or a combination of LPS and NECA for 18 hours. The medium was then removed and the monolayers rinsed with phosphate-buffered saline (PBS) at room temperature. Whole cell extracts were then prepared using 1 ml of RIPA buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) containing 1% protease inhibitor cocktail (product no. P8340, Sigma) and 1 mmol/L sodium orthovanadate at 4°C. The lysates were centrifuged at 10,000 × g for 10 minutes at 4°C. Protein concentrations were determined by the Bradford method using the Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Hercules, CA). Fifty μg of protein were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After transfer onto nitrocellulose membranes, TRAF6 protein was detected using a goat polyclonal antibody from Santa Cruz Inc. The blots were developed using an enhanced chemifluorescence system (GE Health Care, Piscataway, NJ) and visualized using a Typhoon 9410 variable mode imager (GE Health Care). To control for sample loading, blots were also stained with a rabbit polyclonal antibody to nucleoplasmin (Cell Signaling Technology, Beverly, MA).
VEGF Assay
VEGF protein levels in macrophage-conditioned media were assayed using Quantikine M murine VEGF enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. Each sample was assayed for VEGF in duplicate and results are presented as means ± SD.
TNF-α Assay
TNF-α protein levels in macrophage-conditioned media were determined using a sandwich ELISA kit (R&D Systems), as described by the manufacturer. Each sample was assayed in duplicate for TNF-α. Results are presented as means ± SD.
Determination of Macrophage Viability by the MTT Assay
At the end of each experiment, the viability of the cells was assessed by the MTT assay as described previously.30
Preparation of Excisional Skin Wounds in Mice
MyD88+/+ and MyD88−/− were maintained in the barrier facility in the New Jersey Medical School animal care facility under specific pathogen-free conditions. All wounding procedures were performed in a specific pathogen-free surgical suite under a laminar flow hood to ensure sterility and to avoid wound infection. Full thickness wounds were inflicted in the dorsal skin of MyD88+/+ or MyD88−/− female mice, 10 weeks of age (Jackson Laboratory). A total of 56 MyD88−/− and 56 MyD88+/+ mice were used. Each strain of mice was divided into three groups, with equal numbers being assigned to each group. The control group was treated with 1.5% (w/v) carboxymethylcellulose in PBS. The test groups had 200 μg/ml CGS21680, 2.5 mg/ml ZM241385, or 200 μg/ml CGS21680 + 2.5 mg/ml ZM241385, in 1.5% carboxymethylcellulose in PBS. At time = 0 days, a full-thickness, circular 10-mm-diameter wound was created. Mice were anesthetized by isoflurane inhalation, and the dorsal skin was prepared for surgery using Betadine (The Purdue Frederick Co., Norwalk, CT) and 70% isopropanol. Wounds were created using surgical scissors. A digital planimetric image of the wound was recorded using a Pixera video camera and stored on a PC using JPEG format. A calibration scale was recorded with each image. The wounds were then dressed with a transparent dressing (Tegaderm; 3M, St. Paul, MN), which was adhered to the area surrounding the wound using tincture of benzoin. Fifty μl of the control or test solutions were then injected through the dressing onto the wounds. Wounds were redressed daily, and fresh control or test solutions were applied while the mice were under isoflurane inhalation anesthesia. The dressings were removed, and wounds were flushed with sterile saline to remove debris and clean the wound area. A digital planimetric image of the wound was recorded each day, as described above. At various time points after wounding (1, 2, 5, 7, 9, and 12 days), two animals from each group were sacrificed, and the wounds with ∼2 mm of surrounding skin were excised and placed into Streck’s fixative (Streck Labs Inc., Omaha, NE). Wounds were dissected through their center to provide cross-sections through the full width of the wounds. These samples were embedded in paraffin for histological and immunohistochemical analyses, as described below.
Analysis of Wound Areas
Wound areas were analyzed using ImagePro v 5.1. Stored JPEG images were analyzed in Image Pro, and the calibration scale photographed with each wound was used as an internal calibration for the determination of wound areas using the image analysis program. Each wound was independently outlined three times, and at least four wounds were analyzed at each time point.
Histological and Immunohistochemical Analyses
For histological analysis, 5-μm sections were prepared and stained with hematoxylin and eosin (H&E) or with Gomori’s trichrome. For immunohistochemistry, sections were prepared on Superfrost Plus slides (Erie Scientific Co., Portsmouth, NH), deparaffinized with Histoclear (Fisher Scientific Inc., Fairlawn, NJ), and rehydrated through graded ethanols (100%, 90%, 70%, 50%), followed by PBS and water. The sections were then treated with 0.01 mol/L citrate buffer, pH 6.0, in a pressure cooker for 1 minute for antigen recovery and washed with PBS. Endogenous peroxidase was blocked by incubation with 3% hydrogen peroxide in methanol for 30 minutes. Sections were then stained either with anti-CD31 antibody to label the endothelial cells of blood vessels or with anti-F4/80 antibody to label macrophages. After incubation with biotin-labeled secondary antibodies, sections were incubated with streptavidin peroxidase and developed with Nova Red (Vector Laboratories, Burlingame, CA). For quantitation of the area of each wound occupied by newly forming blood vessels, four cross sections from each wound were analyzed using ImageQuant. Control of bias was achieved by assigning codes to each section, which were then analyzed by investigators who were blinded to the identity of each of the groups. The code was broken after the completion of the analyses. For the analysis of macrophage content, F4/80-positive macrophages were counted on at least four cross sections from each wound by investigators blinded to the identity of each group. Determination of cell number per unit area in this way avoids the problems of nonspecific background staining associated with the measurement of F4/80 staining using ImageQuant image analysis.
Statistics
Where appropriate, data are expressed as mean values with their associated SD. Statistical analyses of data were conducted using analysis of variance with Bonferroni posthoc analysis. P values between compared groups less than or equal to 0.05 are considered statistically significant.
Results
TNF-α and VEGF Production by Macrophages from MyD88−/− and MyD88+/+ Mice
To determine the requirement for signaling through the MyD88-dependent signaling pathway in the phenotypic switch of macrophages from production of TNF-α to VEGF, the response of macrophages from 7- to 8-week-old female MyD88 knockout mice (MyD88−/− mice) was compared to that of macrophages from wild-type littermates (MyD88+/+ mice). Macrophages were incubated for 20 hours and left untreated or treated with LPS (100 ng/ml), R848 (1 μmol/L), NECA (1 μmol/L), CGS21680 (1 μmol/L), LPS (100 ng/ml) + NECA (1 μmol/L), CGS21680 (1 μmol/L) + NECA (1 μmol/L), R848 (1 μmol/L) + NECA (1 μmol/L), or R848 (1 μmol/L) + CGS21680 (1 μmol/L). Conditioned media were harvested and analyzed for TNF-α and VEGF content by ELISA. As shown in Figure 1A, both LPS and R848 strongly induced TNF-α expression by MyD88+/+ macrophages. In MyD88−/− macrophages, TNF-α induction by R848 was completely eliminated, and LPS induction of TNF-α was severely diminished. As shown in Figure 1B, VEGF expression was strongly induced in MyD88+/+ macrophages by LPS/NECA and by R848/NECA, whereas LPS and R848 alone had little effect. In contrast, in MyD88−/− macrophages, the synergistic effect of NECA with LPS or R848 was completely lost.
Figure 1.
Macrophages from MyD88−/− mice do not exhibit synergistic up-regulation of VEGF expression in response to TLR agonists and adenosine A2A receptor agonists. Peritoneal macrophages (106 cells/ml) from MyD88+/+ or MyD88−/− mice were treated for 20 hours with LPS (100 ng/ml), R848 (1 μmol/L), CGS21680 (1 μmol/L), NECA (1 μmol/L), LPS + CGS21680, LPS + NECA, R848 + CGS21680, or R848 + NECA. Conditioned media were assayed for TNF-α (A) and VEGF (B) using ELISAs. Each test group was performed in triplicate (n = 3), and each medium sample was assayed in duplicate. Results are expressed as means ± SD and represent the cytokine concentration present in 1 ml of medium conditioned by 106 cells.
TNF-α and VEGF Production by Macrophages from IRAK4−/− and IRAK4+/+ Mice
IRAK4 is a serine-threonine kinase that forms part of the signaling complex associated with TLR signaling though MyD88.36,44 To determine the requirement for IRAK4 in the phenotypic switch of macrophages from production of TNF-α to VEGF, the response of macrophages from 7- to 8-week-old female IRAK4 knockout mice (IRAK4−/− mice) was compared to that of macrophages from wild-type littermates (IRAK4+/+ mice). As shown in Figure 2A, both LPS and R848 induced TNF-α expression in wild-type (IRAK4+/+) mice. Interestingly, the level of TNF-α expression induced by these agonists was markedly higher than that induced in macrophages from control C57BL/6 mice, or macrophages from MyD88+/+ mice. The reason for this difference is unclear but may relate to the particular background strains of these mice. The induction of TNF-α in response to LPS or R848 was abrogated in IRAK4−/− macrophages. The synergistic up-regulation of VEGF in response to LPS or R848 in conjunction with NECA or CGS21680 was similar in wild-type IRAK4+/+ mice to that in MyD88+/+ mice, and this synergistic up-regulation was also abrogated in the IRAK4−/− macrophages (Figure 2B). As we observed with MyD88−/− macrophages, the basal effects of NECA and CGS21680 on VEGF expression were not affected in IRAK4−/− macrophages.
Figure 2.
Macrophages from IRAK4−/− mice do not exhibit synergistic up-regulation of VEGF expression in response to TLR agonists and adenosine A2A receptor agonists. Peritoneal macrophages (106 cells/ml) from IRAK4+/+ or IRAK4−/− mice were treated for 20 hours with LPS (100 ng/ml), R848 (1 μmol/L), CGS21680 (1 μmol/L), NECA (1 μmol/L), LPS + CGS21680, LPS + NECA, R848 + CGS21680, or R848 + NECA. Conditioned media were assayed for TNF-α (A) and VEGF (B) using ELISAs. Each test group was performed in triplicate (n = 3), and each medium sample was assayed in duplicate. Results are expressed as means ± SD and represent the cytokine concentration present in 1 ml of medium conditioned by 106 cells.
TNF-α and VEGF Expression by RAW264.7 Cells and RAW264.7 Cell Clones Transfected with siRNA to Silence TRAF6 Expression
TRAF6 is a signaling molecule recruited to the MyD88-IRAK4 complex that mediates TLR-dependent downstream signaling.35,46,47,48 TRAF6 is an E3 ubiquitin ligase, and on activation by IRAK4, TRAF6 mediates lysine 63 (K63)-linked self-ubiquitination as well as K63-linked ubiquitination of several target proteins, resulting in the triggering of IκB kinase (IKK) and the activation of NF-κB as well as MAP kinase and AP1 signaling pathways.49,50 Although TRAF6-deficient mice have been bred, they show defects in LPS- and IL-1-induced activation of Jnk and NF-κB, are osteopetrotic, and do not generally survive beyond a few weeks of age.51,52 Because we have been unable to obtain these mice, we chose instead to use siRNA depletion of TRAF6 as an alternative approach to analyze the requirement for this gene in the synergistic up-regulation of VEGF expression by LPS and NECA or CGS21680. RAW264.7 cells, a murine macrophage-like cell line, robustly express TNF-α in response to LPS and R848 and also show the synergistic up-regulation of VEGF in response to A2AR agonists. RAW264.7 cells were transfected with siRNA small hairpin loop constructs cloned into the pSilencer 3.1-H1neo vector, and stable clones expressing siRNA were selected using G418 resistance. Control transfectants containing scrambled RNA sequences were also selected. Six clones from each transfection were analyzed for TRAF6 mRNA by TaqMan RT-PCR and for TRAF6 protein by Western blot analysis. Clones transfected with siRNA for TRAF6 that showed at least 70% down-regulation of TRAF6 mRNA and protein in comparison to nontransfected cells or cells transfected with scrambled sequences were selected for further study. TRAF6-silenced clones and control clones were plated in 12-well culture dishes (1 × 106 cells per dish in 1 ml of RPMI-1% FCS) and incubated overnight in RPMI-1% FCS. The cells were then treated with LPS, R848, NECA, CGS21680, LPS/NECA, LPS/CGS21680, R848/NECA, or R848/CGS21680, as described above. After 20 hours of incubation, conditioned media were harvested and assayed for TNF-α and VEGF content. Figure 3, A and B, shows TNF-α and VEGF production, respectively, by these clones. Clones with silenced TRAF6 showed markedly reduced production of TNF-α in comparison to control clones in response to LPS and to R848. Similarly VEGF production in response to LPS/NECA or R848/NECA was markedly reduced, suggesting that TRAF6 signaling is an important mediator of the synergistic response of macrophages to TLR and A2AR ligation.
Figure 3.
Suppression of TRAF6 expression in RAW264.7 macrophages blocks the synergistic up-regulation of VEGF expression in response to TLR agonists and adenosine A2A receptor agonists. RAW264.7 macrophages were transfected with siRNA small hairpin loop constructs cloned into the pSilencer3.1-H1neo vector. TRAF6-specific siRNAs as well as control scrambled sequences were transfected. Stable clones were selected using G418. TRAF6-specific siRNA clones exhibiting at least 70% down-regulation of TRAF6 mRNA and protein expression in comparison to controls were selected for study. Cells (1 × 106/ml) were plated and treated with TLR agonists and/or A2AR agonists for 20 hours. Conditioned media were harvested and analyzed for TNF-α (A) and VEGF (B) content by ELISAs. Each test group was performed in triplicate (n = 3), and each medium sample was assayed in duplicate. Results are expressed as means ± SD and represent the cytokine concentration present in 1 ml of medium conditioned by 106 cells.
Dermal Wound Healing in MyD88−/− versus MyD88+/+ Mice
Figure 4 shows a series of images of excisional wounds from MyD88+/+ mice versus MyD88−/−. Wounds in MyD88+/+ mice contracted and granulated throughout the course of ∼9 days, showing 50% reduction in wound area by ∼3 days after injury. In contrast, wounds in MyD88−/− mice healed at a markedly slower rate. Fifty percent reduction in wound area was observed only after 5 days. By 12 days after injury, most wounds in MyD88−/− mice were still not completely healed. No overt signs of wound infection were observed in any of the wounds. Figure 5 shows the quantified data for wound area of MyD88+/+ wounds versus MyD88−/− wounds throughout the course of 12 days.
Figure 4.
Excisional skin wounds in MyD88−/− mice show delayed wound healing. Full thickness wounds were inflicted in the dorsal skin of 10-week-old female MyD88+/+ or MyD88−/− mice. Wounds were treated with 1.5% (w/v) carboxymethylcellulose in PBS. At 0 time, a full-thickness, 10-mm-diameter wound was created. A digital image of the wound was recorded in JPEG format using a video camera. A calibration scale was recorded with each image. Wounds were dressed with a transparent dressing (Tegaderm, 3M) and were redressed daily. Digital images of the wounds were recorded at the indicated time points, as described above. A, C, E, and G show typical wounds in MyD88+/+ mice; B, D, F, and H show wounds in MyD88−/− mice.
Figure 5.
Quantitative analysis of wound closure in MyD88+/+ and MyD88−/− mice. Video images of wounds in JPEG format were outlined in ImagePro 5.1 and wound area was analyzed. Each wound was independently outlined and analyzed three times. The number of wounds measured at each time point for each test group is indicated. Results are presented as mean areas ± SD. *P < 0.05.
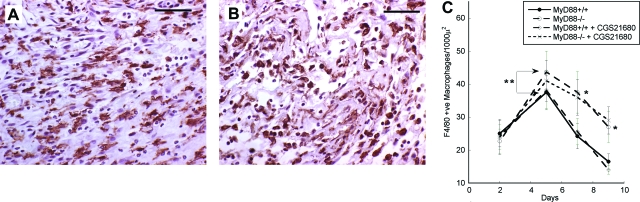
To determine the macrophage content of the wounds, cross-sections were immunostained with F4/80 monoclonal antibody. Figure 6, A and B, shows typical sections of wounds 5 days after injury from MyD88+/+ and MyD88−/− wounds, respectively. F4/80-positive cells were counted on least five sections from each wound, and the numbers of macrophages within five high-power fields (×40 objective) were determined. Macrophage content of wounds in MyD88−/− and MyD88+/+ did not differ significantly at day 2, but in the MyD88−/− wounds the macrophage content was greater at day 5 than that in MyD88+/+ wounds. This increased level persisted throughout the course of the study. Figure 6C shows the macrophage content of the wounds at days 2, 5, 7, and 9.
Figure 6.
Immunohistochemical localization of macrophages in wounds. Five-μm cross-sections through the full width of the wounds were stained with F4/80 anti-macrophage monoclonal antibody as described in the Materials and Methods section. The labeled cells were then visualized using biotin-labeled secondary antibodies, followed by streptavidin-peroxidase. Typical sections of MyD88+/+ (A) and MyD88−/− (B) wounds 5 days after wounding are presented. At least four sections of each wound (n = 2) were stained immunohistochemically with F4/80 anti-macrophage monoclonal antibody and analyzed for macrophage content. At least five high-power fields (×40 objective) within each section were examined. C: Quantitative analysis of macrophage numbers within MyD88+/+ and MyD88−/− wounds. Results are presented as the number of F4/80-stained macrophages per 1000 μ2 ± SD. *P < 0.05 for MyD88−/− versus MyD88+/+ wounds; **P < 0.05 for MyD88+/+ versus MyD88+/+ plus CGS21680. Scale bars = 50 μm.
To analyze the presence and distribution of blood vessels in the healing wounds, cross sections were immunostained with an anti-CD31 antibody. Figure 7, A–D, shows typical views of areas adjacent to the edge of the wounds at days 5 and 9 after wounding in MyD88+/+ (Figure 7, A and C) and MyD88−/− (Figure 7, B and D) mice. Neovascularization in MyD88+/+ control wounds was particularly apparent at the edge of the wounds and at their base, with growth of new vessels occurring in conjunction with fibroblast accumulation and matrix deposition. CD31-positive vessel profiles were quantified using Image-Quant. At least four sections for each wound were analyzed, and the areas within five high-power fields (×40 objective) of the margin of the wounds were examined. In the MyD88−/− wounds, the density of blood vessels was markedly decreased at all stages of healing. The quantitative analysis of blood vessel content in the various wounds is shown in Figure 7E.
Figure 7.
Immunohistochemical localization of blood vessels in wounds. Five-μm cross-sections through the full width of the wounds were stained with polyclonal anti-CD31 (PECAM) antibodies, as described in the Materials and Methods section. The labeled cells were then visualized using biotin-labeled secondary antibodies, followed by streptavidin-peroxidase. Typical sections of MyD88+/+ (A and C) and MyD88−/− (B and D) wounds 5 days and 9 days after wounding are presented. E: Quantitative analysis of CD31-stained blood vessel content in MyD88+/+ and MyD88−/− wounds. Sections of wounds that were stained immunohistochemically with anti-CD31 polyclonal antibodies were analyzed for blood vessel content using ImagePro 5.1. At least four sections from each wound (n = 2) were measured. Areas within five high-power fields (×40 objective) of the wound margins were examined, and profiles staining positively with the anti-CD31 antibody were quantitated. Results are presented as the mean percentage of wound area occupied by CD31-stained blood vessels ± SD. *P < 0.05 for MyD88−/− versus MyD88+/+ wounds; **P < 0.05 for MyD88+/+ versus MyD88 plus CGS21680. Scale bars = 50 μm.
Effect of CGS21680 on Wound Healing in MyD88−/− versus MyD88+/+ Mice
Treatment of excisional skin wounds in normal mice with the A2AR agonist CGS21680 has been shown previously to enhance wound healing, accelerating the rate of closure and increasing formation of granulation tissue and neovascular blood vessel density.32,40,41 These results were confirmed in this study. Figure 8A shows the effect of CGS21680 on wound closure in MyD88+/+ mice. CGS21680-treated wounds in these mice showed an accelerated rate of closure in comparison to vehicle-treated wounds that was statistically significant at days 5, 7, and 9 after wounding. The A2AR antagonist ZM241385 had little effect alone on the rate of wound closure but antagonized the promotional effects of CGS21680, returning the wound closure rate to that of controls. In contrast, treatment of wounds in MyD88−/− mice with CGS21680 had no significant effect on wound closure, with CGS21680-treated wounds in MyD88−/− mice healing at the same rate as control wounds in these mice (Figure 8B). This indicates that the stimulatory effect of CGS21680 observed in normal mice is lost in the knockout mice.
Figure 8.
Effects of the adenosine A2AR agonist CGS21680 on wound closure in MyD88+/+ and MyD88−/− mice. Full thickness wounds were inflicted in the dorsal skin of 10-week-old female MyD88+/+ or MyD88−/− mice. Controls were treated with 1.5% (w/v) carboxymethylcellulose in PBS. Test groups had 200 μg/ml CGS21680, 2.5 mg/ml ZM241385, or 200 μg/ml CGS21680 + 2.5 mg/ml ZM241385, in 1.5% carboxymethylcellulose in PBS. At 0 time, a full thickness, 10-mm-diameter wound was created. A digital image of the wound was recorded in JPEG format using a video camera. A calibration scale was recorded with each image. Wounds were dressed with a transparent dressing (Tegaderm, 3M). Fifty μl of the control or test solutions were injected through the dressings onto the wounds. Wounds were redressed daily and fresh control or test solutions applied. Digital images of the wounds were recorded at the indicated time points, as described above. Video images of wounds in JPEG format were outlined in ImagePro 5.1 and the wound area was analyzed. Each wound was independently outlined and analyzed three times. The number of wounds measured at each time point for each test group is indicated. Results are presented as mean areas ± SD. A: Wound areas in MyD88+/+ mice. B: Wound areas in MyD88−/− mice. *P < 0.05.
Treatment with CGS21680 did not significantly alter the macrophage content of either MyD88+/+ or MyD88−/− wounds (Figure 6C). The minor reduction in macrophage content induced by CGS21680 in MyD88−/− wounds at day 5 did not approach statistical significance. No significant differences in granulation tissue formation, macrophage content, or blood vessel density were observed between MyD88−/− control wounds and CGS21680-treated MyD88−/− wounds. However, wild-type (MyD88+/+) wounds treated with CGS21680 showed significantly increased density of blood vessels in granulation tissue compared to wounds treated with vehicle alone (Figure 7E). This increase was observed at 5 and 7 days after wounding. Similar results have been reported previously.32,33,40,41
Measurement of VEGF and HIF1-α mRNA Levels in MyD88+/+ and MyD88−/− Wounds
To determine whether the expression of VEGF and HIF1-α mRNAs was altered in wounds of MyD88+/+ versus MyD88−/− mice, total RNA was extracted from wounds 4 and 8 days after wounding. mRNA levels were also determined in uninjured skin from the same mice, using skin samples taken from areas at least 1 cm removed from the wound site. Four mice per group were wounded for these analyses. The levels of VEGF and HIF1-α mRNAs were determined by quantitative RT-PCR and normalized to the levels of cyclophilin-D, a housekeeping gene. The relative levels of VEGF and HIF1-α mRNAs in the skin and wounds are shown in Table 3. VEGF mRNA levels in uninjured skin were low and did not differ significantly between wild-type and knockout mice. At day 4 after wounding, VEGF mRNA levels in wounds were elevated in the wounds of MyD88+/+ mice compared to those in MyD88−/− mice. This difference was more marked at day 8. HIF1-α mRNA levels were elevated above that of uninjured skin in day 4 MyD88+/+ wounds and further increased at day 8. In MyD88−/− wounds, there was no significant increase in HIF1-α mRNA in day 4 wounds. By day 8, however, HIF1-α mRNA levels in MyD88−/− wounds increased, reaching levels similar to those of MyD88+/+ wounds at day 4. These levels were still significantly lower than those of MyD88+/+ wounds at day 8. These results suggest that in MyD88−/− mice there is a deficit in the induction of HIF1-α mRNA and that VEGF is induced to a lesser degree than in MyD88+/+ mice.
Table 3.
Relative Levels of VEGF and HIF1-α mRNA in Wounds of MyD88+/+ and MyD88−/− Mice
| VEGF mRNA
|
HIF1-α mRNA
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 4
|
Day 8
|
Day 4
|
Day 8
|
|||||
| Skin | Wound | Skin | Wound | Skin | Wound | Skin | Wound | |
| MyD88+/+ | 20 ± 2 | 80 ± 7 | 15 ± 1.8 | 150 ± 17 | 2323 ± 227 | 3492 ± 487 | 2256 ± 241 | 4133 ± 373 |
| MyD88−/− | 17 ± 1.8 | 67 ± 5.2* | 14 ± 1.3 | 100 ± 9* | 2309 ± 211 | 2147 ± 198* | 2177 ± 220 | 3524 ± 281* |
Wounds (n = 4) were sampled at each time point for the preparation of total RNA. mRNA levels for VEGF and HIF1-α were determined using Q-RT-PCR as described in Materials and Methods. Skin samples were taken from uninjured skin from an area at least 1 cm removed from the wound sites. All values are normalized to cyclophilin-D.
P < 0.05 relative to MyD88+/+ levels.
Discussion
TLRs are expressed at high levels on macrophages and act as pattern recognition receptors for a wide range of components of foreign organisms.53 Ligand binding to TLRs initiates signaling cascades that activate macrophages to express inflammatory mediators, including chemokines and cytokines. This activation equips the cells with the armamentarium required to destroy and eliminate these organisms. TLR signaling is mediated by the interaction of their cytoplasmic TIR domains with adaptor proteins, which include MyD88, TIRAP (MAL), TRIF, and TRAM.17,18,20,54 Most TLRs require MyD88 as a critical cytoplasmic adaptor; TLR4 signals through both MyD88 as well as through a MyD88-independent, TRIF/TRAM-dependent pathway, and TLR3 signals independently of MyD88, through a TRIF-dependent pathway.16,35,54,55
We have shown previously that signaling in macrophages through TLRs 2, 4, 7, and 9, which classically results in up-regulated expression of inflammatory cytokines such as TNF-α and IL-12, is dramatically altered in the presence of adenosine receptor ligands.28,30 Adenosine, acting through A2A and A2B receptors, strongly down-regulates the expression of TNF-α and IL-12 and, through A2A receptors, strongly up-regulates the expression of the angiogenic growth factor VEGF.25,27,28,56 We have termed this alternative pathway of macrophage activation an “angiogenic switch.”28 Other cytokines and chemokines, including IL-10 and MIP1-α, are also strongly regulated by this switch.26,27,29,57 In this study we have examined the requirement for the downstream signaling molecules MyD88, IRAK4, and TRAF6 in this angiogenic switch. Using macrophages from mice deficient in MyD88 (MyD88−/− mice), we tested the effects of the TLR4 agonist Escherichia coli LPS and the TLR2 agonist PAM3SK4 in the absence or presence of the adenosine A2AR agonists CGS21680 or NECA on the production of TNF-α and VEGF. As expected, TNF-α production was greatly diminished in MyD88−/− macrophages in response to LPS or PAM3SK4. In addition, the synergistic up-regulation of VEGF induced by these agonists in the presence of A2AR agonists was totally eliminated. This indicates clearly that MyD88 is an essential signaling intermediate in this angiogenic switch.
MyD88 acts by recruiting several proteins into a signaling complex,58 and a key protein of this complex is the interleukin-1 receptor-associated kinase 4 (IRAK4).12,16,36 IRAK4 associates with MyD88 and with IRAK1, resulting in the recruitment to the complex of TNF receptor-associated factor-6 (TRAF6).35,48,50 TRAF6 is an E3 ubiquitin ligase, which is activated by lysine-63 (K63)-mediated self ubiquitination.8,50,59 The K63-ubiquitinated TRAF6 then mediates K63-linked ubiquitination of downstream targets, including receptor-interacting protein (RIP) and the IKK subunit NEMO (IKK-γ). This then mediates activation of the TAB/TAK complex, which in turn activates AP1-, MAP kinase-, or NF-κB-dependent signaling pathways. In macrophages from IRAK4-deficient mice, we find that induction of TNF-α expression by LPS or PAM3SK4 is greatly reduced and that the synergistic up-regulation of VEGF in the presence of A2AR agonists is also abrogated. This indicates that IRAK4 is also a critical mediator of this signaling pathway. To date, we have been unable to obtain TRAF6-deficient mice; however, using siRNA technology, we have prepared stable clones of the RAW264.7 macrophage-like cell line that respond to TLR agonists and adenosine receptor agonists in a manner analogous to that of primary macrophages from mice. In these experiments we selected clones that exhibited >70% silencing of TRAF6 at both mRNA and protein levels (data not shown) and compared the response of these cells and control cells transfected with scrambled, nonspecific siRNA constructs to LPS, PAM3SK4, and the A2AR agonists CGS21680 and NECA. TRAF6-silenced clones showed a markedly decreased production of TNF-α in response to LPS or PAM3SK4. They also showed a markedly decreased expression of VEGF in response to LPS or PAM3SK4 with CGS21680 or NECA, indicating that TRAF6 is also an important intermediate in the TLR/A2AR-mediated angiogenic switch. The signaling events downstream of TRAF6 that regulate VEGF expression are not yet known.
We have shown previously that LPS-induced activation of NF-κB is unaffected by adenosine receptor ligation, although TNF-α protein production is strongly suppressed.30,57 We have also shown previously that the synergistic induction of VEGF expression by LPS with A2AR agonists critically requires transcriptional induction through binding of the HIF1 transcription factor to the hypoxia-response-element in the VEGF promoter.31,60 This up-regulation of HIF involves a strong increase in steady state levels of HIF1-α mRNA. It seems likely, therefore that adenosine signaling modulates TLR signaling by inducing an alternative, as yet unidentified, pathway downstream of TRAF6 that leads to the up-regulation of HIF1-α expression. It is interesting to note in this regard that TRAF6 has recently been shown to be an interacting partner of β-arrestins, which are important modulators of G-protein-coupled receptors such as the A2AR, thus providing a potential link between TLR signaling and adenosine signaling.61
MyD88-deficient mice (MyD88−/− mice) have been shown previously to develop normally.43,61,62,63 However, these mice show a decreased susceptibility to the development of atherosclerotic lesions when bred onto the apoE−/− background and fed a high-fat diet.64,65 MyD88−/− mice also show defective intestinal re-epithelialization in a model of intestinal injury,66,67 and macrophages have been implicated in this defect. In this study, we examined the role of MyD88 in the healing of excisional skin wounds in mice. Wounds in MyD88−/− mice were found to heal at a markedly slower rate than wounds in wild-type MyD88+/+ mice. This delayed healing was characterized by slower contraction, a decreased rate of formation of granulation tissue, and a decreased density of blood vessels in the newly forming granulation tissue. At the early stages of repair, macrophages were present in MyD88−/− and MyD88+/+ wounds at comparable levels; however, by day 5 after wounding, macrophages accumulated in the MyD88−/− wounds to higher levels than in MyD88+/+ controls and persisted at higher levels throughout the study. No evidence of infection was observed in any of the wounds. These results suggest that signaling through the MyD88-dependent pathway plays an important role in the regulation of wound healing. Macrophages are known to play an important role in the regulation of angiogenesis by producing angiogenic factors such as VEGF. Production of VEGF by macrophages is strongly regulated by TLR signaling, in conjunction with adenosine A2AR signaling.28,30 We show here that MyD88, a principal mediator of TLR signaling, is critically required in vitro for the synergistic up-regulation of VEGF expression by macrophages in the presence of A2AR agonists and that loss of MyD88 abrogates the production of VEGF by this pathway. Despite the increased accumulation of macrophages in vivo in MyD88−/− wounds, neovascularization in granulation tissue was reduced, suggesting that the lack of MyD88 signaling prevents the up-regulation of the expression of angiogenic growth factors such as VEGF in these wounds. However, although macrophages are a major cell type expressing MyD88 in wounds, enabling a strong response to TLR agonists, other cells also express TLRs and MyD88, and it is not clear from our in vivo experiments that the delayed wound-healing response in MyD88−/− mice is attributable solely to defective macrophage signaling. Resolution of this question will require the selective deletion of MyD88 or A2AR from specific cell populations, for example by bone marrow transfer or by the use of cre-lox technology, to determine whether MyD88 expression in local stromal cells, such as endothelial or epithelial cells, or in bone marrow-derived cells, is responsible for the defects in wound repair.
A2ARs are broadly expressed on a wide range of cell types other than macrophages. Neutrophils are prominent cells present at the early stages of wound healing and express both A2ARs and TLRs. No obvious changes in the levels of neutrophils between MyD88+/+ and MyD88−/− wounds were observed in these experiments (data not shown). However, A2ARs are known to play a role in the regulation of neutrophil function,68,69,70 and the absence of MyD88 signaling in these cells could play a role in the wound-healing deficits we observe in MyD88−/− mice. Also in this context, we have previously studied other cell types, including skin keratinocytes, fibroblasts, and microvascular endothelial cells in vitro to determine whether the TLR/A2AR-dependent angiogenic switch is operative in cells other than macrophages. Keratinocytes and fibroblasts did not exhibit this response, and endothelial cells did not respond to LPS and A2AR agonists (unpublished data). However, we have observed synergistic interactions in endothelial cells stimulated with IL-1, TNF-α, and A2AR agonists, indicating that cells other than macrophages that play a critical role in inflammation and wound healing may also be modulated by the interaction of MyD88 and A2AR signaling.71
There has been considerable interest recently in alternatively activated macrophage phenotypes, with classically activated (M1) macrophages being implicated in the regulation of inflammation and alternatively activated M2 macrophages participating in the regulation of angiogenesis.72,73,74,75,76,77,78 Acquisition of the M2 phenotype was originally proposed to require stimulation by glucocorticoids and cytokines such as IL-10 and IL-13.73 We have proposed that the TLR/adenosine-mediated angiogenic switch provides a simple, physiologically relevant, mechanism for switching macrophages to an M2-like phenotype, in a manner that does not rely on the presence of particular cytokines, but only on the accumulation of extracellular adenosine, which occurs as a consequence of hypoxia and ischemia.30,79 As noted above in MyD88−/− wounds, macrophages accumulate to a higher level than in MyD88+/+ wounds and persist at higher levels in these wounds. Because MyD88−/− macrophages have reduced NF-κB-dependent signaling and produce less inflammatory cytokines, it is possible that the reduced capacity of these cells to participate in inflammation and wound debridement is in part responsible for the delayed wound healing response in these mice. In addition, the failure of CGS21680, an adenosine A2AR agonist, to stimulate wound repair and angiogenesis in these wounds suggests that the switch of macrophages from an inflammatory (M1) to an angiogenic (M2) phenotype is also defective and that macrophages in these wounds remain in either an unstimulated or in an M1-like inflammatory phenotype, failing to switch to an angiogenic M2-like phenotype.
A role for adenosine signaling in wound healing has been demonstrated previously, and adenosine A2ARs have been shown to be important regulators of angiogenesis and wound healing.25,28,30,32,33,34,40,60,80,81 Mice lacking A2ARs show impaired wound healing characterized by delayed wound closure, delayed and reduced angiogenesis, and granulation tissue formation. Treatment of skin wounds in mice and rats with A2AR agonists such as CGS21680 or MRE0094 accelerates wound healing, with a prominent effect on wound angiogenesis.33,34 A2AR agonists are currently in clinical trials for the treatment of chronic wounds. Because A2AR signaling synergizes with TLR signaling to switch macrophages from the production of TNF-α to the production of VEGF, we tested whether the effects of A2AR agonists in vivo in promoting wound healing are dependent on signaling through MyD88. Wounds in MyD88−/− and MyD88+/+ mice were treated with CGS21680, an adenosine A2AR-specific agonist. In MyD88+/+ mice, CGS21680 induced a significant acceleration of the rate of wound closure and an increase in the density of new blood vessels in the forming granulation tissue. This effect was antagonized by ZM241385, a specific A2AR antagonist.82 In contrast, in MyD88−/− mice, CGS21680 had no significant accelerating effect and did not affect blood vessel density, and these wounds still exhibited an impaired healing phenotype. This suggests that the effects of CGS21680 in vivo depend on the presence of an intact MyD88 signaling system and that in the absence of MyD88 the effects of CGS21680 on the induction of angiogenesis are lost.
Because the angiogenic switch mediated by co-ligation of TLRs 2, 4, 7, or 9 and adenosine A2ARs involves the up-regulation of VEGF expression in a HIF1-α-dependent manner,31 we used quantitative RT-PCR to analyze whether expression of VEGF and HIF1-α differed in MyD88+/+ and MyD88−/− wounds. At day 4 after wounding, VEGF mRNA levels were mildly elevated in wounds of MyD88+/+ mice compared to those in MyD88−/− mice. This difference was more marked at day 8. VEGF mRNA levels in uninjured skin were low and did not differ between wild-type and knockout mice. HIF1-α mRNA levels were significantly elevated over those in uninjured skin at day 4 in wounds from MyD88+/+ but not from MyD88−/− mice. At day 8 after wounding, HIF1-α mRNA levels were elevated in MyD88−/− wounds to approximately the same level as those in day 4 MyD88+/+ wounds but were lower than those in MyD88+/+ wounds at day 8. These results suggest that in MyD88−/− mice there is a deficit in the induction of HIF1-α mRNA and that VEGF is induced to a lesser degree than in MyD88+/+ mice. Further studies will be required to determine whether the decreased expression of HIF1-α and VEGF mRNAs in MyD88−/− wounds is a feature of reduced expression in macrophages alone, or whether other cell types in the wound also show decreased expression of these factors. It should be noted here that the synergistic action of LPS with A2AR agonists involves transcriptional induction of VEGF in a pathway that requires the up-regulation of HIF1-α mRNA and protein expression, in a hypoxia-independent manner.31 Hypoxia, on the other hand, induces HIF1-α protein stabilization without the induction of increased HIF1-α mRNA. Within the context of healing wounds, both hypoxia- and adenosine-mediated pathways are likely to be active. The lack of MyD88-dependent signaling may block the adenosine-mediated induction of HIF1-α mRNA but will not block hypoxia-dependent HIF1-α protein stabilization. Determining the relative contributions of these pathways will require additional studies.
LPS, a TLR4 agonist, has been shown recently to strongly induce the expression of A2ARs on macrophages (M. Ramanathan, W. Lus, and S.J. Leibovich, manuscript submitted).83 Inflammatory cytokines such as IL-1 and TNF-α, whose expression is induced in macrophages by the action of TLR agonists, also modulate the expression and function of A2ARs on macrophages.84 It is possible that in MyD88−/− mice the lack of MyD88, which is a critical mediator of TLR-dependent signaling, prevents up-regulation of A2AR expression and function by macrophages after wounding, thus abrogating responsiveness to A2AR agonists. We are currently testing this hypothesis.
An intriguing and unanswered question on the role of MyD88 in the regulation of wound healing relates to the nature of the agonist(s) that stimulates signaling through the MyD88 pathway. Signaling through the IL-1R family, by IL-18 and by IL-33 through the ST2R, as well as by a variety of TLRs, stimulates MyD88 signaling.16,42,85 Although a role for IL-1 in wound healing has been proposed, IL-1R knockout mice exhibit retarded oral but not dermal wound healing.86,87,88 Studies of wound healing in IL-18, IL-33, or ST2 knockout mice have not been reported. Wounds in TLR4-deficient mice do not exhibit defective healing,89 suggesting that signaling through MyD88-dependent receptors other than TLR4 might play an important role in wound healing.17,20,62,90,91,92,93,94 Although the wounds in our experiments were inflicted under aseptic conditions and mice were maintained under specific pathogen-free conditions, there are numerous commensal organisms that reside asymptomatically in and on the body, and it is possible that some of these organisms play a beneficial role in triggering TLRs in response to wounding, thus acting symbiotically rather than commensally.67,68,95,96,97 Alternatively, several endogenous agonists of TLRs have been reported, including HSP70, alternatively spliced isoforms of fibronectin, fragments of hyaluronic acid, oxidized lipids, and others.94,98 The ligands and receptors required for stimulation of MyD88 signaling in wound repair remain to be elucidated.
In summary, we have found that the synergistic up-regulation of VEGF expression in macrophages in vitro critically requires signaling through MyD88, IRAK4, and TRAF6. In vivo, MyD88-deficient mice show a markedly impaired wound-healing phenotype, characterized by delayed wound closure and granulation tissue formation, with a decreased density of new blood vessels. A role for adenosine A2AR signaling in this wound-healing impairment in MyD88-deficient mice is implied by the failure of CGS21680, an A2AR-specific agonist, to stimulate wound healing in MyD88−/− mice. This suggests that the synergistic regulation of macrophage phenotype by TLR agonists with A2AR agonists that we have demonstrated in vitro may also play a role in vivo by regulating wound-healing responses.
Acknowledgments
We thank Dr. S. Akira, Tokyo, Japan, for the kind gift of MyD88 knockout mice; and Dr. Wen-Chen Yeh, Toronto, Canada, for the kind gift of IRAK4 knockout mice.
Footnotes
Address reprint requests to Dr. Samuel Joseph Leibovich, Department of Cell Biology and Molecular Medicine, The Cardiovascular Research Institute, New Jersey Medical School, UMDNJ, 185 South Orange Ave., Newark, NJ 07103. E-mail: leibovic@umdnj.edu.
Supported by the United States Public Health Service (grants RO1-GM068636 to S.J.L., and R01GM56268, AA13336, and AR41911 to B.N.C.), King Pharmaceuticals (to B.N.C.), and the Plastic Surgery Educational Foundation (fellowship to L.M.).
Although none of the authors of this article have a competing financial interest, Dr. Cronstein holds a patent on the use of adenosine A2A receptor agonists for wound healing applications and has received funding from King Pharmaceuticals, Inc. to study the effects of an adenosine A2A receptor agonist (MRE0094) in wound healing. The patent on use of adenosine A2A receptor agonists to promote wound healing has been assigned to New York University School of Medicine and is licensed for development to King Pharmaceuticals. King Pharmaceuticals is a large (market capitalization >$3 billion) publicly traded corporation and has an agent in phase II clinical trials for wound healing indications. Dr. Cronstein has served as a consultant for these studies. Dr. Cronstein is also a member of the scientific advisory board of CanFite Biopharmaceuticals and holds stock in that company.
References
- Leibovich SJ, Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976;84:501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich SJ, Ross R. The role of the macrophage in wound repair: a study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;84:71–85. [PMC free article] [PubMed] [Google Scholar]
- DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–240. [PubMed] [Google Scholar]
- Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–627. [PubMed] [Google Scholar]
- Polverini PJ. Macrophage-induced angiogenesis: a review. Cytokines. 1989;1:54–73. [Google Scholar]
- Fraser I, Doyle A, Hughes D, Gordon S. Use of surface molecules and receptors for studying macrophages and mononuclear phagocytes. J Immunol Methods. 1994;174:95–102. doi: 10.1016/0022-1759(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Gordon S. Macrophage-restricted molecules: role in differentiation and activation. Immunol Lett. 1999;65:5–8. doi: 10.1016/s0165-2478(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Brown GD, Geldhof AB, Martinez-Pomares L, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–2097. doi: 10.1002/eji.200324003. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol. 2002;270:81–92. doi: 10.1007/978-3-642-59430-4_5. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Nicholson AC, Han J, Febbraio M, Silverstein RL, Hajjar DP. Role of CD36, the macrophage class B scavenger receptor, in atherosclerosis. Ann NY Acad Sci. 2001;947:224–228. doi: 10.1111/j.1749-6632.2001.tb03944.x. [DOI] [PubMed] [Google Scholar]
- Stahl PD. The mannose receptor and other macrophage lectins. Curr Opin Immunol. 1992;4:49–52. doi: 10.1016/0952-7915(92)90123-v. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther. 2005;7:12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SN, Fitzgerald KA, Fenton MJ. TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol Interv. 2003;3:466–477. doi: 10.1124/mi.3.8.466. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hemmi H, Akira S. Interferon response induced by Toll-like receptor signaling. J Endotoxin Res. 2004;10:252–256. doi: 10.1179/096805104225005896. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells. Circ Res. 2002;90:531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol. 2004;16:237–270. doi: 10.1615/critrevneurobiol.v16.i4.20. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Adenosine and endothelial cell function. Hasko G, Cronstein BN, Szabo C, editors. Boca Raton,: CRC Taylor and Francis,; 2006:pp 109–130. [Google Scholar]
- Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Haskó G, Kuhel DG, Chen JF, Schwarzschild A, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A2A receptors. Am J Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C, Scott GS, Virag L, Egnaczyk G, Salzman AL, Shanley TP, Hasko G. Suppression of inflammatory protein (MIP)-1alpha production and collagen-induced arthritis by adenosine receptor agonists. Br J Pharmacol. 1998;125:379–387. doi: 10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, Fink JS, Cronstein B. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A2A receptor agonists and endotoxin. Am J Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan M, Pinhal-Enfield G, Hao I, Leibovich SJ. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2A receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element (HRE) in the VEGF promoter. Mol Biol Cell. 2007;18:14–23. doi: 10.1091/mbc.E06-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A2A receptors. Am J Pathol. 2002;160:2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN. Adenosine receptors and wound healing. Sci World J. 2004;4:1–8. doi: 10.1100/tsw.2004.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohda J, Matsumura T, Inoue J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not Toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol. 2004;173:2913–2917. doi: 10.4049/jimmunol.173.5.2913. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Yeh WC. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol. 2002;23:503–506. doi: 10.1016/s1471-4906(02)02298-6. [DOI] [PubMed] [Google Scholar]
- O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Han J. MyD88 beyond Toll. Nat Immunol. 2006;7:370–371. doi: 10.1038/ni0406-370. [DOI] [PubMed] [Google Scholar]
- Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor-Vega C, Desai A, Montesinos MC, Cronstein BN. Adenosine A2A receptor agonists promote more rapid wound healing than recombinant human platelet-derived growth factor (Becaplermin gel). Inflammation. 2002;26:19–24. doi: 10.1023/a:1014417728325. [DOI] [PubMed] [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanashi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1 and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Duncan GS, Millar DS, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, Penninger JM, Wesche H, Ohashi PS, Mak TW, Yeh WC. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–754. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- Ramanathan M, Giladi A, Leibovich SJ. Regulation of vascular endothelial growth factor expression in murine macrophages by nitric oxide and hypoxia. Exp Biol Med. 2003;228:697–705. doi: 10.1177/153537020322800608. [DOI] [PubMed] [Google Scholar]
- Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-kappa B by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappa B-inducing kinase—identification of a novel TRAF6 interaction motif. J Biol Chem. 1999;274:7724–7731. doi: 10.1074/jbc.274.12.7724. [DOI] [PubMed] [Google Scholar]
- Pollet I, Opina CJ, Zimmerman C, Leong KG, Wong F, Karsan A. Bacterial lipopolysaccharide directly induces angiogenesis through TRAF6-mediated activation of NF-kappaB and c-Jun N-terminal kinase. Blood. 2003;102:1740–1742. doi: 10.1182/blood-2003-01-0288. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes Infect. 2004;6:1333–1338. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito A, Yoshida H, Nishioka E, Satoh M, Azuma S, Yamamoto T, Nishikawa S, Inoue J. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci USA. 2002;99:8766–8771. doi: 10.1073/pnas.132636999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creagh EM, O’Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Mak TW, Sen G, Li X. Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci USA. 2004;101:3533–3538. doi: 10.1073/pnas.0308496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Szabo C. Regulation of monocyte/macrophage function by adenosine receptors. Hasko G, Cronstein BN, Szabo C, editors. Boca Raton,: CRC Taylor and Francis,; 2006:pp 49–68. [Google Scholar]
- Nemeth ZH, Leibovich SJ, Deitch EA, Vizi ES, Szabo C, Hasko G. cDNA microarray analysis reveals a nuclear factor-kB-independent regulation of macrophage function by adenosine. J Pharmacol Exp Ther. 2003;306:1042–1049. doi: 10.1124/jpet.103.052944. [DOI] [PubMed] [Google Scholar]
- Rawlings DJ. The biology and biochemistry of inflammatory signalosomes. Meeting on signaling networks in immunity and inflammation. EMBO Rep. 2006;7:25–30. doi: 10.1038/sj.embor.7400599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S, Sanjo H, Akira S, Matsumoto K, Ninomiya-Tsuji J. TAK1-binding protein 2 facilitates ubiquitination of TRAF6 and assembly of TRAF6 with IKK in the IL-1 signaling pathway. Genes Cells. 2005;10:447–454. doi: 10.1111/j.1365-2443.2005.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich SJ. Regulation of macrophage-dependent angiogenesis by adenosine and Toll-like receptors. Hasko G, Cronstein BN, Szabo C, editors. Boca Raton,: CRC Taylor and Francis,; 2006:pp 325–346. [Google Scholar]
- Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- Feng CG, Scanga CA, Collazo-Custodio CM, Cheever AW, Hieny S, Caspar P, Sher A. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J Immunol. 2003;171:4758–4764. doi: 10.4049/jimmunol.171.9.4758. [DOI] [PubMed] [Google Scholar]
- Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- Björkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985;135:1352–1366. [PubMed] [Google Scholar]
- Revan S, Montesinos MC, Naime D, Landau S, Cronstein BN. Adenosine A2 receptor occupancy regulates stimulated neutrophil function via activation of a serine/threonine protein phosphatase. J Biol Chem. 1996;271:17114–17118. doi: 10.1074/jbc.271.29.17114. [DOI] [PubMed] [Google Scholar]
- Khoa ND, Postow M, Danielsson J, Cronstein BN. Tumor necrosis factor-alpha prevents desensitization of Galphas-coupled receptors by regulating GRK2 association with the plasma membrane. Mol Pharmacol. 2006;69:1311–1319. doi: 10.1124/mol.105.016857. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Deepak P, Kumar S, Acharya A. Interleukin-13-induced type II polarization of inflammatory macrophages is mediated through suppression of nuclear factor-kappaB and preservation of IkappaBalpha in a T cell lymphoma. Clin Exp Immunol. 2007;149:378–386. doi: 10.1111/j.1365-2249.2007.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guo JJ, Su FX, Yao HR, Chen JS. Alternatively activated macrophages/mononuclear phagocytes promote growth and invasion of breast cancer cell line SKBR3. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:410–413. [PubMed] [Google Scholar]
- Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, Mollenkopf H, Kaufmann SH. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36:631–647. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ. Macrophages, Adenosine and Toll-Like Receptors: Their Role in the Regulation of Atherosclerotic Lesion Development. Reiss AB, Carsons S, Cronstein BN, editors. Kerala,: Research Signpost,; 2006:pp 35–62. [Google Scholar]
- Adair TH. An emerging role for adenosine in angiogenesis. Hypertension. 2004;44:618–620. doi: 10.1161/01.HYP.0000144802.18301.2f. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Keddie JR, Poucher SM, Shaw GR, Brooks R, Collis MG. In vivo characterisation of ZM 241385, a selective adenosine A2A receptor antagonist. Eur J Pharmacol. 1996;301:107–113. doi: 10.1016/0014-2999(96)00020-9. [DOI] [PubMed] [Google Scholar]
- Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoa ND, Montensinos MK, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Macrophage differentiation marker Myd88 is a member of the Toll/IL-1 receptor family. Biochem Biophys Res Commun. 1994;199:144–146. doi: 10.1006/bbrc.1994.1206. [DOI] [PubMed] [Google Scholar]
- Mertz PM, Sauder DL, Davis SC, Kilian PL, Herron AJ, Eaglstein WH. IL-1 as a potent inducer of wound reepithelialization. Prog Clin Biol Res. 1991;365:473–480. [PubMed] [Google Scholar]
- Liu W, Ding I, Chen K, Olschowka J, Xu J, Hu D, Morrow GR, Okunieff P. Interleukin 1beta (IL1B) signaling is a critical component of radiation-induced skin fibrosis. Radiat Res. 2006;165:181–191. doi: 10.1667/rr3478.1. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Kimura A, Matsushima K, Mukaida N. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J Immunol. 2006;176:5598–5606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- Bettinger DA, Pellicane JV, Tarry WC, Yager DR, Diegelmann RF, Lee R, Cohen IK, DeMaria EJ. The role of inflammatory cytokines in wound healing: accelerated healing in endotoxin-resistant mice. J Trauma. 1994;36:810–813. doi: 10.1097/00005373-199406000-00010. [DOI] [PubMed] [Google Scholar]
- Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci. 2002;27:474–482. doi: 10.1016/s0968-0004(02)02145-x. [DOI] [PubMed] [Google Scholar]
- Chen Q, Carroll HP, Gadina M. The newest interleukins: recent additions to the ever-growing cytokine family. Vitam Horm. 2006;74:207–228. doi: 10.1016/S0083-6729(06)74008-0. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity. 2005;23:461–462. doi: 10.1016/j.immuni.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Gordon JI. A genomic view of our symbiosis with members of the gut microbiota. J Pediatr Gastroenterol Nutr. 2005;40(Suppl 1):S28. doi: 10.1097/00005176-200504001-00016. [DOI] [PubMed] [Google Scholar]
- Xu J, Gordon JI. Inaugural article: honor thy symbionts. Proc Natl Acad Sci USA. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]