Abstract
In crescentic glomerulonephritis (GN), monocyte chemoattractant protein-1 (MCP-1) is overexpressed within the glomeruli, and MCP-1 blockade has renoprotective effects. Adult podocytes are in a quiescent state, but acquisition of a migratory/proliferative phenotype has been described in crescentic GN and implicated in crescent formation. The cognate CC chemokine receptor 2 (CCR2), the MCP-1 receptor, is expressed by other cell types besides monocytes and has been implicated in both cell proliferation and migration. We investigated whether MCP-1 binding to CCR2 can induce a migratory/proliferative response in cultured podocytes. MCP-1 binding to CCR2 enhanced podocyte chemotaxis/haptotaxis in a concentration-dependent manner and had a modest effect on cell proliferation. Closure of a wounded podocyte monolayer was delayed by CCR2 blockade, and CCR2 was overexpressed at the wound edge, suggesting a role for CCR2 in driving podocyte migration. Immunohistochemical analysis of kidney biopsies from patients with crescentic GN demonstrated CCR2 expression in both podocytes and cellular crescents, confirming the clinical relevance of our in vitro findings. In conclusion, the MCP-1/CCR2 system is functionally active in podocytes and may be implicated in the migratory events triggered by podocyte injury in crescentic GN and other glomerular diseases.
Podocytes are highly differentiated cells with a complex cellular morphology. The podocyte cell body bulges into the urinary space and gives rise to primary processes that extend toward the capillaries to which they affix by numerous foot processes. The foot process of neighboring podocytes interdigitate, leaving between them filtration slits bridged by an extracellular structure, known as the slit diaphragm, which represents the major restriction site to protein filtration.1
In the adult kidney, podocytes are in a quiescent state; however, both proliferation and acquisition of a migratory phenotype have been reported in pathological conditions. In crescentic glomerulonephritis (GN), podocytes detach from the glomerular basement membrane (GBM), assume a migratory phenotype, and trigger crescent formation by establishing bridges between the tuft and the Bowman’s capsule.2 In addition, cells derived from migrated podocytes proliferate and participate in crescent formation.3 Furthermore, in nephrotic conditions, podocyte effacement, which requires cytoskeleton remodeling, foot process movement over the GBM, and slit diaphragm reconstruction, may also be considered a migratory event aimed to compensate for podocyte loss by covering areas of bare GBM.4
Monocyte chemoattractant protein-1 (MCP-1) is a potent mononuclear cell chemoattractant produced by a variety of mesenchymal cells, including glomerular cells.5,6,7 Within the glomeruli, there is MCP-1 overexpression in both crescent GN8 and nephrotic conditions.9,10,11 Furthermore, immunohistochemistry studies have shown that glomerular podocytes are the predominant glomerular cell type overexpressing MCP-1 in various proteinuric conditions, such as diabetic, hypertensive, and membranous nephropathies.9,12,13 Finally, recent studies have shown that blockade/loss of MCP-1 has antiproteinuric and renoprotective effects in both experimental diabetic nephropathy14 and crescentic GN,15,16 suggesting a role of MCP-1 in the pathogenesis of the glomerular damage.
MCP-1 binds and signals through a seven-transmembrane protein-coupled receptor, the cognate CC chemokine receptor 2 (CCR2), which is predominantly expressed by monocytes.17 Local recruitment of monocytes is considered the predominant mechanism by which MCP-1 contributes to the renal damage; however, CCR2 expression has been demonstrated in other cell types, both in vitro18,19,20,21,22,23 and in vivo,24,25,26,27 indicating that the MCP-1/CCR2 system has other effects beyond monocyte accrual. Notably, MCP-1 binding to the CCR2 receptor induces both chemotaxis and proliferation in epithelial, endothelial, and vascular smooth muscle cells,19,20,21,22,27 suggesting a role of the MCP-1/CCR2 system in conferring a proliferative and migratory phenotype in cells other than monocytes.
In vitro data on CCR2 expression by podocytes are conflicting,28,29 but a recent study in the Alport mouse model has shown overexpression of the CCR2 receptor in glomerular podocytes in vivo,29 suggesting a potential pathophysiological relevance of the MCP-1/CCR2 system in this cell type. The present study was designed to test first whether cultured human podocytes express the CCR2 receptor and whether MCP-1 binding to CCR2 can induce a migratory/proliferative response in this cell type. Second, to determine whether these in vitro findings were relevant in vivo, we have assessed CCR2 expression in renal biopsies from patients with crescentic GN.
Materials and Methods
All materials were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Fetal calf serum (FCS) was from Euroclone (Milan, Italy). Dulbecco’s modified Eagle’s medium and Alexa 555-conjugated streptavidin were from Invitrogen (San Giuliano Milanese, Italy). RNeasy Mini spin columns, RNase-free DNase I, and Taq polymerase were purchased from Qiagen (Milan, Italy). The reverse transcription system was from Promega (Madison, WI) and the Cell Proliferation Bromodeoxyuridine (BrdU) Colorimetric Assay was from Roche Diagnostics (Basel, Switzerland). Oligonucleotide primers, rh-MCP-1, mouse anti-MCP-1, Quantikine human MCP-1/CCL2 immunoassay, and mouse anti-CCR2 antibodies were obtained from R&D Systems (Minneapolis, MN). The rabbit anti-CCR2 and the isotype-specific control antibodies were from Epitomics (Burlingame, CA). The mouse anti-synaptopodin antibody and the polyclonal guinea pig anti-nephrin antibody were from Progen Biotechnik (Heidelberg, Germany), and the fluorescein isothiocyanate-conjugated rat anti-mouse antibody from BD Biosciences (San Jose, CA). The LSAB+ system-HRP, the FITC-conjugated rabbit anti-mouse antibody, and the biotinylated swine anti-rabbit antibody were from DAKO (Glostrup, Denmark). Lymphoprep was from Nycomed Pharma AS (Oslo, Norway), and the polycarbonate filters were from NTG Srl (Milan, Italy).
Cell Culture
Primary cultures of human podocytes were established as previously described.30 Cells were cultured in Dulbecco’s modified Eagle’s medium containing l-glutamine, 6.8 mmol/L glucose, 10% heat-inactivated FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified 5% CO2 incubator at 37°C. Studies were performed at passages 20 to 30. Cell viability was determined by trypan blue exclusion test.
Monocytes were isolated from total blood samples of human healthy volunteers using Lymphoprep density gradient centrifugation followed by adhesion separation in Dulbecco’s modified Eagle’s medium containing 10% FCS. Cell number was determined using a standard counting chamber after cell harvesting with 0.25% trypsin and 0.5% EDTA.
Podocyte Characterization
The cells were identified by positive staining for nephrin, synaptopodin, Wilm’s tumor antigen, podocalyxin, zonula occludens-1, cytokeratin, vimentin, and laminin; negative staining for smooth muscle-type myosin, FVIIIr:Ag, and CD45; and cytotoxicity in response to puromycin aminonucleoside (10 to 50 μg/ml).30 Immunofluorescence staining for podocyte cell markers was performed on cells fixed in 3.5% paraformaldehyde containing 2% sucrose for 15 minutes at 4°C and incubated with polyclonal primary antibodies and then with FITC-conjugated secondary antibodies. To assess cell morphology, scanning electron microscopy was performed on samples postfixed in 2.5% glutaraldehyde, dehydrated in alcohol, dried, and coated with gold by sputter coating. The specimens were examined in a scanning Jeol T300 electron microscope (JEOL, Tokyo, Japan), and images were obtained via secondary electron at a working distance of 15 to 25 mm and at an accelerating voltage of 20 to 25 kV.
RT-PCR Analysis
Total RNA was extracted using RNeasy Mini spin columns and freed from contaminant DNA by treatment with DNase I. RNA was reverse transcribed (1 μg) according to standard protocols. The PCR analysis was performed with oligonucleotide primers specifically designed to amplify human CCR2 (patented by R&D Systems) yielding a PCR product of 406 bp. After an initial denaturation at 94°C for 9 minutes, the cDNA was amplified for 32 cycles with the following setting: denaturation at 94°C for 45 seconds, annealing at 55°C for 45 seconds, and elongation at 72°C for 45 seconds with a final elongation at 72°C for 10 minutes.18 Total RNA obtained from human monocytes, the cell type predominantly expressing CCR2, was used as positive control. PCR products were resolved in a 1.5% agarose gel containing ethidium bromide, and a digital image of the gel was captured using the Gel Doc XR system (Bio-Rad, Milan, Italy).
Cytofluorimetric Analysis
Cells were detached from plates using a nonenzymatic cell dissociation solution and washed with PBS containing 4% heat-inactivated human serum. After blocking with whole heat-inactivated human serum for 15 minutes, cells were incubated with either a mouse anti-human CCR2 or an IgG1 control antibody diluted in PBS containing 4% serum for 45 minutes at 4°C. After washing in PBS containing 4% serum, cells were incubated with a FITC-conjugated rabbit anti-mouse antibody. Cells were then washed twice and analyzed by flow cytometry (FACscan; Becton-Dickinson, Mountain View, CA). For each determination, 10,000 cells were analyzed.
Chemotaxis Assay and Checkerboard Analysis
Chemotaxis assays were performed in standard Boyden chambers with 8-μm pore-size polycarbonate filters precoated with collagen type IV for 2 hours. A podocyte cell suspension in serum-free medium was placed in the upper chamber. Serum-free media containing either rh-MCP-1 (0.1, 1, 10, and 100 ng/ml) or vehicle was added to the lower compartment of the Boyden chamber. In a subset of experiments, the assay was performed on podocytes preincubated with either the specific CCR2 inhibitor RS102895 (RS, 6 μmol/L) or vehicle. After 5 hours of incubation at 37°C, the filters were removed; the migrated cells were fixed in methanol, stained with 2% Giemsa solution, and counted in 10 fields under light microscopy. All conditions were evaluated in duplicate for each experiment. To differentiate between chemokinesis and chemotaxis, checkerboard analysis was performed by adding rh-MCP-1 to both the lower and upper compartment of the Boyden chamber.
Haptotaxis Assay
A haptotaxis assay was performed in triplicate using a Boyden chamber. Briefly, uncoated polycarbonate filters were floated on serum-free media containing either rh-MCP-1 (10 ng/ml) or control (1 mg/ml bovine serum albumin) overnight at 37°C. After washing in PBS, the filters were placed in the Boyden chamber with the coated surface of the filter facing toward the lower well, which was filled with serum-free media.31 Podocytes were then seeded into each upper chamber in serum-free media and incubated for 5 hours at 37°C. At the end of the incubation period, filters were removed, fixed, and stained, and migrated cells were counted as described above.
In Vitro Wound Healing Assay
Podocytes were seeded into 96-well tissue culture plates and allowed to grow to confluence. After a 24-hour quiescent period in medium containing 0.5% FCS, cells were incubated for 1 hour with RS (6 μmol/L) or vehicle, then the medium was removed, and monolayers were wounded using a single pass with a sterile yellow pipette tip. The medium containing either vehicle or rh-MCP-1 (10 ng/ml), with or without RS (6 μmol/L) addition, was returned to the wells, and wound closure was monitored over time with a ×4 objective on a Leica DFC320 phase contrast inverted microscope (Leica, Wetzlar, Germany). Images of the entire wounded area were captured using a Leica DMIL digital camera, and the area of the wound measured in arbitrary units using the Image J 1.32 software (available for free download from http://rsb.info.nih.gov/ij/). This method of injury led to a consistent baseline wounded area (91,273 ± 2853 arbitrary units, n = 21). Each condition was examined in triplicate.
Agarose Strip Method
A modified agarose strip method was used as previously described.32 In brief, a 2.5% agarose and 1% glycerol solution was pipetted as a thin strip on the base of 6-cm plates and allowed to dry. A podocyte cell suspension was added and cultured to form a confluent monolayer interrupted by the agarose strip. The agarose strip was carefully removed, RS (6 μmol/L) or vehicle was added to the wells, and the wound closure was analyzed as described above. All experiments were performed at least in triplicate.
Proliferation Assay
Cell proliferation was assayed using a colorimetric immunoassay based on the measurement of BrdU incorporation according to the manufacturer’s instructions. Briefly, podocytes were plated on flat-bottom 96-well tissue culture plates (density, ∼2500 cells per well) and allowed to adhere overnight. After a 24-hour quiescent period in medium containing 0.5% FCS, rh-MCP-1 (1 to 10 ng/ml) was added to the medium for 12, 24, 48, and 72 hours. Cells were labeled with BrdU during the last 4 hours of the incubation period and fixed, and BrdU incorporation was assayed via colorimetric detection using a plate reader (Bio-Rad 680 Microplate Reader) at 450 nm.
Immunocytochemical Analysis
CCR2 and MCP-1 expression was assessed in podocytes by immunocytochemistry. Cells were fixed in 3.5% paraformaldehyde containing 2% sucrose for 30 minutes. After blocking, cells were incubated with a rabbit anti-human CCR2 antibody, a mouse anti-MCP-1 antibody or isotype-specific irrelevant antibodies. After rinsing, specific staining was revealed using the LSAB+ system-HRP system. Briefly slides were incubated with a biotinylated anti-rabbit secondary antibody, followed by incubation with peroxidase-labeled streptavidin and development using a 3,3′-diaminobenzidine chromogen solution. After counterstaining, slides were mounted and visualized with an Olympus Bx4 I microscope connected to a Leica DMIL camera.
MCP-1 Protein Measurement
Culture supernatants from both wounded and control monolayers were collected, centrifuged to remove cell debris, and stored at −70°C for analysis. MCP-1 protein concentration was measured by a quantitative sandwich enzyme-linked immunosorbent assay using a mouse monoclonal and a rabbit polyclonal anti-human MCP-1 antibody (range, 5 to 2000 pg/ml; intra- and interassay CV, 4.9 and 4.8%, respectively). Results were corrected for cell numbers.
Analysis of Renal Biopsies from Patients with Crescent Glomerulonephritis
The study was performed on renal biopsies of eight patients (five males and three females; age, 63 ± 15 years) with clinical features of rapidly progressive GN and severe renal failure that required hemodyalitic treatment in two patients (serum creatinine 8.2 ± 2.7 mg/ml). Biopsies included in the study presented classic histological features of diffuse crescentic GN. Histological and immunopathological diagnosis was pauci-immune crescentic glomerulonephritis in six cases (that was idiopathic in four cases and secondary to necrotizing vasculitis in two cases), type I membranoproliferative GN in one case, and membranous GN in one case. No patients received steroids or immunosuppressive drugs before renal biopsy. Normal portions of kidneys from patients who underwent surgery for hypernephromas were used as control. The study was approved by the Ethical Committee of the Department of Internal Medicine of the University of Genoa, procedures were in accordance with the Helsinki Declaration, and informed consent was obtained from all subjects.
Immunohistochemical staining was performed on 4-μm paraffin sections of formalin-fixed tissue. Briefly, sections were dewaxed, rehydrated, and immersed in 0.01 mol/L citrate buffer at 100°C for retrieval of antigen sites masked by formalin fixation. Endogenous peroxidase activity was quenched by incubation with 3% H2O2. Endogenous avidin-binding activity was inhibited by sequential treatment with avidin-biotin and nonspecific binding sites blocked with 3% bovine serum albumin. For immunodetection, sections were incubated for 1 hour at room temperature with a rabbit monoclonal anti-CCR2 antibody, and the specific staining was detected using the LSAB+ system-HRP. Sections were counterstained and visualized with an Olympus-Bx4I microscope connected to a Leica DMIL camera. The specificity of the anti-CCR2 antibody was confirmed by replacing the primary antibody with a nonimmune isotypic control antibody. Evaluation of glomerular staining was performed by a pathologist in a blinded fashion.
Double immunofluorescent staining was performed for CCR2 and synaptopodin, a specific podocyte marker. After blocking with 3% bovine serum albumin, sections were incubated with a mouse monoclonal anti-synaptopodin antibody for 18 hours at 4°C, washed in PBS, and then incubated with a FITC-conjugated rat anti-mouse antibody for 1 hour. After washing and further blocking in avidin-biotin, sections were incubated with a monoclonal rabbit anti-CCR2 antibody for 1 hour at room temperature, washed in PBS, incubated with a biotinylated swine anti-rabbit IgG for 1 hour and then with Alexa 555-conjugated streptavidin. Sections were examined using an Olympus epifluorescence microscope (Olympus Bx4 I) with photographic attachment (Leica DMIL). The images were color-combined and assembled into photomontages by using Adobe Photoshop (Universal Imaging Corporation, West Chester, PA).
Data Presentation and Statistical Analysis
The number of experiments, performed at least in triplicate, is reported in the figure legends. All data are presented as mean ± SEM. Data are expressed as fold change over control. Student’s t-test was used for the comparison between two groups. When more than two groups were studied, data were analyzed by analysis of variance, and if significant, the Newman-Keuls was used for post hoc comparisons. Values for P < 0.05 were considered significant.
Results
Podocyte Morphology and Nephrin Expression
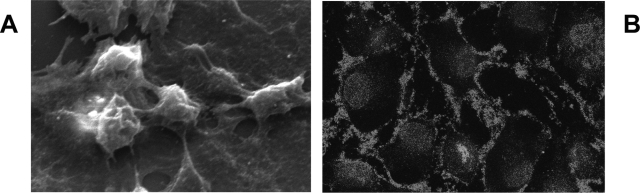
Our cells displayed positive staining for podocyte markers, including peripheral expression of the podocyte-specific marker nephrin. Furthermore, in subconfluent monolayers, scanning electron microscopy demonstrated the presence of the typical cytoplasmatic projections (Figure 1).
Figure 1.
Characterization of human podocytes. A: Representative scanning electron micrograph of podocytes showing typical cytoplasmatic projections. Original magnification, ×1200. B: Podocyte staining for nephrin by immunofluorescence. Original magnification, ×630.
Human Podocytes Constitutively Express the CCR2 Receptor
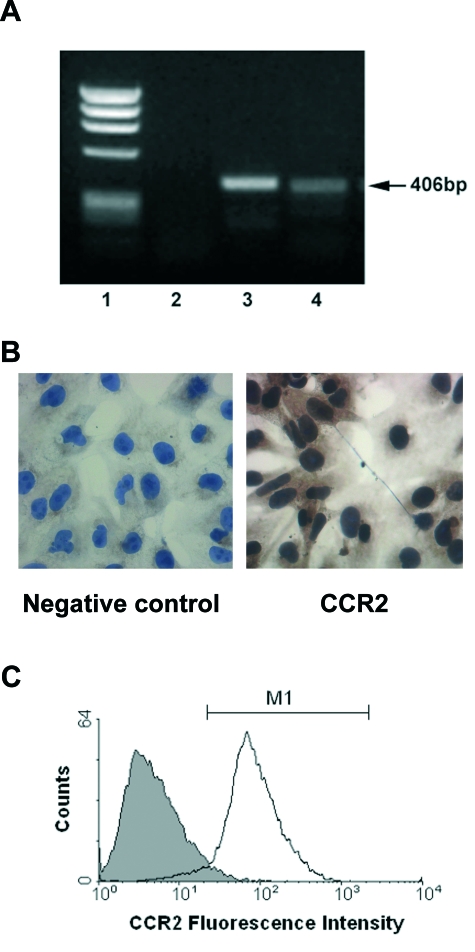
Human podocytes constitutively express the mRNA encoding the CCR2 receptor, as determined by RT-PCR (Figure 2A). A single PCR product of 406 bp was obtained as predicted from the known cDNA sequence for CCR2. Contamination of genomic DNA was excluded by DNase I treatment of total RNA. The specificity of the band was confirmed by the absence of signal in the negative control and by the presence of a band of identical molecular weight in the positive control obtained by amplification of cDNA from human monocytes. Immunocytochemical staining for CCR2 showed positive staining in the majority of podocytes (Figure 2B). Furthermore, surface CCR2 protein expression was demonstrated by cytofluorimetry using an antibody recognizing the external domain of CCR2. CCR2 expression was present in approximately 94% of the podocyte population (Figure 2C).
Figure 2.
CCR2 mRNA and protein is expressed by human podocytes. CCR2 mRNA expression was analyzed by RT-PCR and protein expression by cytofluorimetry and immunocytochemistry in cultured human podocytes as described in Materials and Methods. A: Representative 1.5% agarose gel stained with ethidium bromide. 1, molecular weight marker Φ174-HaeIII. 2, negative control. 3, positive control human monocytes. 4, human podocytes. B: Representative immunocytochemical staining of podocytes for CCR2 (right). The primary antibody was omitted in the negative control (left). Original magnification, ×400. C: Representative histogram showing surface CCR2 expression in podocytes (white histogram). Nonspecific staining (gray histogram) was determined using an isotype-specific control antibody.
MCP-1 Binding to CCR2 Induces Podocyte Chemotaxis
To investigate whether podocytes, besides expressing CCR2, display a functional response to MCP-1, cells were exposed to increasing rh-MCP-1 concentrations (0.1, 1, 10, and 100 ng/ml), and their migration was tested in a chemotaxis assay.
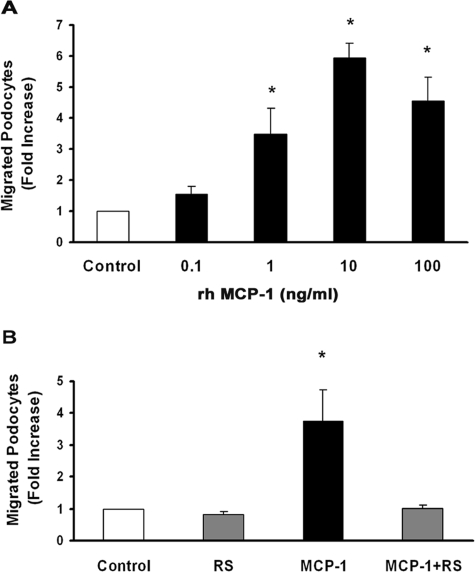
Addition of rh-MCP-1 to the lower chamber enhanced podocyte migration with a significant increase at a dose as low as 1 ng/ml. Maximal induction was observed at 10 ng/ml without further increases at 100 ng/ml (Figure 3A). Because the maximum response was observed at 10 ng/ml, this concentration was used in all subsequent experiments.
Figure 3.
MCP-1 binding to CCR2 induces podocyte chemotaxis. A chemotaxis assay was performed as described in Materials and Methods. Numbers of migrated cells per high-power field were counted, and results are expressed as fold increase over control. A: Podocytes were seeded in the upper chamber of a Boyden chamber, and increasing rh-MCP-1 concentrations (0.1, 1, 10, and 100 ng/ml) were placed in the lower chamber (n = 3). *P < 0.01 rh-MCP-1 at 1, 10, and 100 ng/ml versus control. B: Podocytes were preincubated with either the CCR2 antagonist RS102895 (6 μmol/L) or vehicle for 30 minutes, and then the chemotaxis assay was performed using rh-MCP-1 (10 ng/ml) as chemoattractant (n = 4). *P < 0.05 MCP-1 versus others.
To test whether podocyte migration was a specific effect of MCP-1, experiments were performed in the presence and in the absence of RS (6 μmol/L), added 60 minutes before rh-MCP-1. This compound, which belongs to the spiropiperidine family, interacts specifically with the CCR2-binding domain and has no significant inhibitory activity on other chemokine receptors.33 The addition of RS completely abolished MCP-1-induced podocyte chemotaxis, confirming that the effect was specific and mediated via the CCR2 receptor (Figure 3B). CCR2 blockade did not alter basal podocyte random movement of control cells, suggesting that MCP-1 specifically affects podocyte directional migration. A checkerboard analysis was also performed to determine whether podocyte migration was due to either chemotaxis or chemokinesis. As shown in Table 1, in the absence of MCP-1 in the upper well, podocytes migrated toward MCP-1 placed in the lower well. On the contrary, when equal concentrations of MCP-1 were added to both upper and lower wells to destroy the gradient, very little podocyte migration was observed, indicating that the migratory activity of MCP-1 was directional rather than random in nature.
Table 1.
Checkerboard Analysis of the Effects of MCP-1 on Podocyte Migration
| Lower well | Upper well
|
|
|---|---|---|
| 0 | 10 | |
| 0 | 4.4 ± 0.8 | 4.4 ± 2.1 |
| 10 | 19.3 ± 3.8* | 3.1 ± 0.5 |
Number of podocytes migrated in 5 hours from the upper to the lower well of the Boyden chambers, which were filled with Dulbecco’s modified Eagle’s medium containing 0.5% BSA with or without MCP-1 (10 ng/ml) as indicated. Mean ± SEM per high power field.
P < 0.001 versus others.
MCP-1 Binding to CCR2 Induces Podocyte Haptotaxis
Given that podocyte motility is an adhesion-dependent event, we also investigated the ability of substrate-bound MCP-1 to induce podocyte migration. As shown in Figure 4, rh-MCP-1 bound to the polycarbonate filters of the chemotaxis chamber and induced a significant threefold increase in podocyte migration in the absence of a soluble chemoattractant. Furthermore, MCP-1-induced podocyte haptotaxis occurred via the CCR2 receptor because it was completely blocked by podocyte preincubation with RS.
Figure 4.
MCP-1 induces podocyte haptotaxis via the CCR2 receptor. Podocytes were seeded in the upper chamber of a Boyden chamber and a chemotaxis assay performed using polycarbonate filters precoated with either 10 μg/ml rh-MCP-1 or bovine serum albumin (1 mg/ml) in the presence or the absence of RS102895 (6 μmol/L) or vehicle as described in Materials and Methods. Numbers of migrated cells per high-power field were counted, and results are expressed as fold increase over control (n = 3). *P < 0.01 rh-MCP-1 versus others.
CCR2 Blockade Delays the Closure of a Wounded Podocyte Monolayer
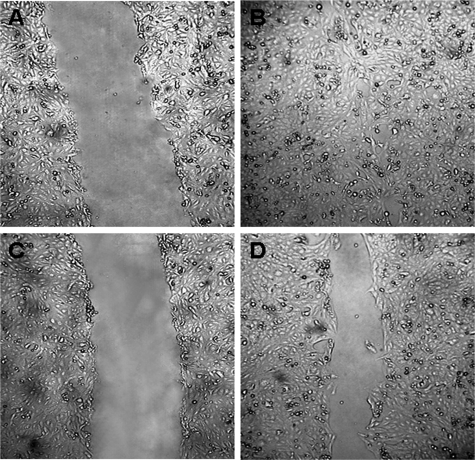
To test whether the MCP-1-induced migratory phenotype could be implicated in the healing of a wounded podocyte monolayer, we used an in vitro scrape-wound assay model.34 In preliminary experiments, we found that wounded podocyte monolayers underwent almost complete closure within 24 hours as assessed by light microscopy. The addition of the CCR2 inhibitor RS significantly delayed podocyte wound healing compared with vehicle at 24 hours (control versus RS, 12 ± 3 versus 38.1 ± 2.4%; percent wound area compared with baseline; P < 0.001) (Figure 5), indicating that CCR2 participates in the normal closure of mechanical wounds in podocyte cultures. This effect was not due to RS cytotoxicity because there were no differences in cell viability between podocytes exposed to RS and control cells (control versus RS: 24 hours, 98.8 ± 0.021 versus 98.7 ± 0.91%; 48 hours, 99.2 ± 0.81 versus 99.7 ± 0.28%; percent viable cells; P = NS). To assess whether podocyte damage was important for inducing CCR2-dependent healing, experiments were also performed using an agarose strip method that introduces gaps into the monolayers without causing significant perturbation to cells. In the presence of RS, a comparable 25% difference in the residual wounded area was observed at 24 hours also in this model (P < 0.05), suggesting that podocyte loss rather than podocyte damage was involved. MCP-1 concentration was similar in the conditioned media from wounded and control monolayers (63.7 ± 1.4 versus 49 ± 6.5 pg/ml; P = NS), and the addition of exogenous rh-MCP-1 to wounded monolayers did not further increase the rate of closure of the mechanical wound (control versus MCP-1, 12 ± 3 versus 18 ± 0.2%; P = NS).
Figure 5.
CCR2 blockade delays the closure of a mechanically wounded podocyte monolayer. Podocyte confluent monolayers were wounded as described in Materials and Methods. Experiments were performed in the presence of RS102895 (6 μmol/L) (C and D) or vehicle (A and B). Wounded area was photographed at baseline (A and C) and after 24 hours (B and D). Representative images are shown of four independent experiments. Original magnification, ×40.
Effect of MCP-1 on Podocyte Proliferation
Because MCP-1 has mitogenic activity in both vascular smooth muscle and endothelial cells, we tested in podocytes whether MCP-1 causes an increase in proliferation that could contribute to CCR2-dependent accelerated wound closure. Podocytes were exposed to rh-MCP-1 (1 to 10 ng/ml) for various time periods (12, 24, 48, and 72 hours), and then proliferation was assessed by both cell counting and BrdU incorporation. As depicted in Figure 6, a significant 25% increase in the number of counted cells was observed in podocytes exposed to 10 ng/ml MCP-1 for 48 hours. Similarly, podocyte exposure to rh-MCP-1 (10 ng/ml) for 48 hours induced a significant 29% increased in BrdU incorporation. No changes in cell proliferation were seen at other time points or MCP-1 concentrations.
Figure 6.
Effect of MCP-1 on podocyte cell number. Quiescent podocytes were exposed to rh-MCP-1 at concentrations of 1 ng/ml (□) and 10 ng/ml (▪) for 12, 24, 48, and 72 hours. Cell proliferation was assessed by cell counting and expressed as fold increase over control (dashed line) (n = 5). *P < 0.05 MCP-1 at 10 ng/ml versus control at 48 hours.
Podocytes Adjacent to the Wounded Area Overexpress the CCR2 Receptor
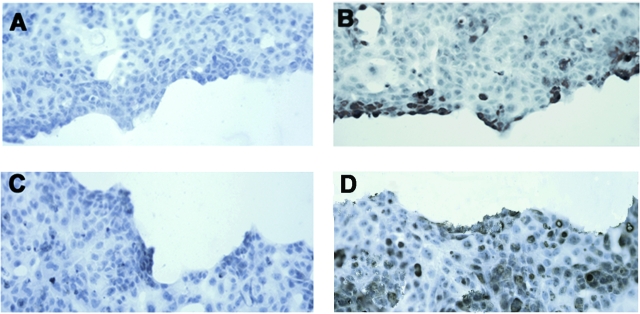
To clarify the mechanism whereby CCR2 blockade delays wound closure, wounded podocyte monolayers were stained for CCR2 and MCP-1 by immunocytochemistry. As shown in Figure 7, podocytes adjacent to the wounded area stained positive for CCR2, whereas the intensity of the staining was lower in cells positioned in areas away from the site of injury. No differences were seen in the staining for MCP-1. In these experiments, we used immunocytochemistry conditions (ie, shorter duration of substrate incubation) aimed to enhance differences in staining distribution/intensity, and this explains the lower basal staining for CCR2 in comparison with cells shown in Figure 2B.
Figure 7.
Injured podocytes overexpress the CCR2 receptor. CCR2 (B) and MCP-1 (D) expression was assessed in wounded podocyte monolayers by immunocytochemistry as described in the Materials and Methods. A and C: Negative controls: incubation with irrelevant isotype-specific control antibodies. Representative images are shown of three independent experiments. Original magnification, ×40.
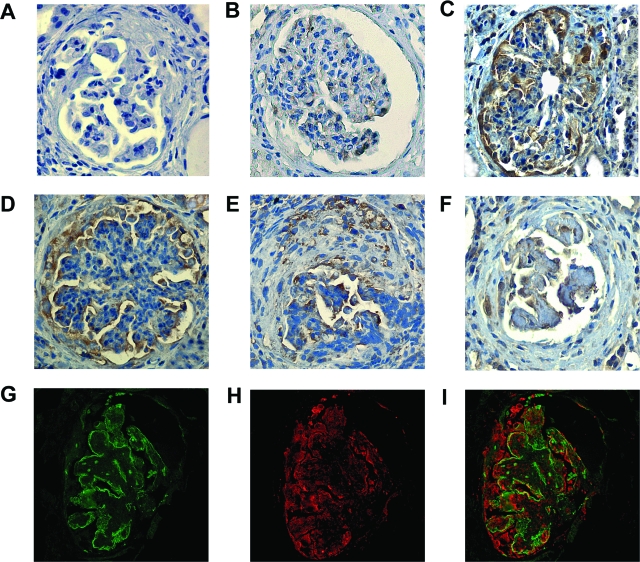
CCR2 Is Overexpressed in Renal Biopsies from Patients with Crescent Glomerulonephritis
To assess the in vivo relevance of our findings and to exclude that CCR2 receptor expression was solely related to in vitro culture conditions, we studied by immunohistochemistry glomerular CCR2 expression in renal sections from control subjects and patients with crescentic GN. We found that only a few glomerular cells, predominantly podocytes, stained positively for CCR2 in the normal glomeruli (Figure 8B). CCR2 staining was greatly enhanced in patients with crescentic GN and localized to the glomerular podocytes, Bowman’s capsule, and the cellular crescents (Figure 8, C–F). The specificity of the antibody binding in immunohistochemistry was confirmed by the disappearance of the signal when an isotype-matched control antibody was used as primary antibody (Figure 8A). Double-labeling immunofluorescence for the podocyte marker synaptopodin (Figure 8G) and CCR2 (Figure 8H) showed colocalization (Figure 8I), confirming CCR2 receptor by glomerular podocytes. CCR2-positive and synaptopodin-negative cells were present in the crescents.
Figure 8.
Glomerular CCR2 staining in patients with crescentic glomerulonephritis. Glomerular staining for CCR2 by immunohistochemistry in a normal kidney (B) and kidney biopsies from patients with crescentic glomerulonephritis (C–F). Nonspecific staining was determined using a nonimmune isotypic control antibody (A). Double immunofluorescence for synaptopodin (G) and CCR2 (H) showed partial colocalization of the positive staining, as demonstrated by merging (I). Magnification, ×400.
Discussion
In the present study, we have demonstrated that human differentiated podocytes express a functionally active CCR2 receptor and that MCP-1 binding to CCR2 induces a migratory phenotype in this cell type. Furthermore, we have provided evidence that the CCR2 receptor is also expressed in vivo in human crescentic GN by glomerular podocytes.
Our results demonstrate that podocytes express the CCR2 receptor at both the mRNA and protein level. A previous report failed to demonstrate mRNA encoding for CCR2 in podocytes,28 whereas a recent study has shown the CCR2 receptor in murine podocytes.29 Our data confirm this latter finding in cells of human origin; furthermore, they provide evidence that CCR2 is functionally active because exposure to MCP-1 induced a significant sixfold increase in podocyte migration. Although originally believed to be a chemoattractant solely for monocytes, there is now evidence that MCP-1 induces migration of several other cell types,19,21,22 and our data extend this to human podocytes.
The peak effect of MCP-1 on podocyte migration was seen at a dose of 10 ng/ml. This concentration is within the higher physiological range because it is comparable with that measured both in vitro in podocytes exposed to high glucose concentrations35 and in vivo at sites of inflammation.36 Addition of exogenous MCP-1, however, is superimposed to the MCP-1 endogenously produced by podocytes and thus mimics a state of MCP-1 excess, suggesting that superphysiological MCP-1 levels are required to induce migration. MCP-1-induced chemotaxis declined at 100 ng/ml, a phenomenon also reported for MCP-1-induced migration of murine mesangial cells.37 A bell-shaped dose-response curve is often noted with chemokine responses and presumably reflects desensitization of the response after treatment with high chemokine concentrations.
The complete inhibition of MCP-1-induced podocyte chemotaxis by RS102895 indicates a specific MCP-1 effect occurring via the CCR2 receptor. The CCR2 receptor exists in two isoforms, CCR2A and CCR2B.17 The latter is responsible for all of the known biological effects of MCP-1, whereas the significance of the CCR2A variant has not yet been elucidated.38 In this study, CCR2 was inhibited using RS102895, a potent and specific antagonist of the CCR2B receptor33; thus the isoform B is likely to be implicated.
Assessment of migration in the presence and the absence of a concentration gradient of MCP-1 showed that the migration was directed and not solely due to an increase in random movement. In addition, in the haptotaxis assay, podocyte migration was significantly enhanced when MCP-1 was bound to the filter. This may suggest that MCP-1 secretion followed by binding to matrix components, laid down by the cell front, creates a natural concentration gradient to direct migration.
Covering areas of denudated GBM is a critical event in podocyte pathophysiology, and an MCP-1-induced migratory phenotype could be implicated in this repairing process. Our results provide evidence that closure of a wounded podocyte monolayer is significantly delayed by the addition of RS102895. Monolayers underwent almost complete healing within 24 hours, but there was a significant 26% reduction in the rate of closure in the presence of RS102895. Similar results were obtained using a method that introduces gaps into the cell monolayers without causing significant perturbation to cells, indicating that podocyte damage is not necessary for the induction of motility but rather that the induction of movement is the result of the sudden availability of tissue culture surface area on which the cells can move. Previous studies have reported a role of the MCP-1/CCR2 system in wound closure in other cell types, such as type II alveolar and bronchial epithelial cells19,22; however, to our knowledge, this is the first report implicating the MCP-1/CCR2 system in directing the migration to efficient wound healing in podocytes. Of interest, the addition of exogenous rh-MCP-1 did not accelerate wound closure, a finding previously reported in alveolar epithelial cells.19 This is unlikely to be due to saturation of the system by endogenously produced MCP-1 because we found a relatively low MCP-1 concentration in the supernatant of wounded podocyte monolayers.
Repair occurs by both cell proliferation and migration. In our study, the MCP-1 effect on podocyte proliferation was quite modest and required 48 hours to occur. It is thus unlikely that CCR2 blockade interferes with wound closure by preventing podocyte proliferation, and inhibition of podocyte migration is a more likely mechanism. Although podocytes are considered terminally differentiated cells unable to undergo proliferation in vivo, recent studies have documented that podocytes are capable of proliferation in various glomerular diseases,39,40 including crescent GN,41 suggesting a potential pathophysiological relevance of our in vitro finding showing a podocyte proliferative response to MCP-1. Similarly, a mitogenic activity of MCP-1 has been reported in vascular smooth muscle cells and recently implicated in both intimal hyperplasia and accelerated atherosclerosis in vivo.20,42
To clarify the mechanism whereby CCR2 blockade delays wound closure, wounded podocyte monolayers were stained for both CCR2 and MCP-1 by immunocytochemistry. Results showed that the intensity of the staining for CCR2 was greater in podocytes adjacent to the wounded area compared with those located in areas away from the site of injury; whereas no changes were seen in MCP-1 staining. Furthermore, MCP-1 concentrations were similar in the conditioned media from wounded and control podocyte monolayers. Altogether, these data support the hypothesis that a localized CCR2 overexpression is the key determinant of podocyte directional migration rather than changes in MCP-1 expression/ concentration.
To assess whether these in vitro findings are relevant to in vivo pathophysiological conditions, we have studied by immunohistochemistry CCR2 expression in both normal renal cortex and kidney biopsies from patients with crescentic GN. In the normal kidney, we found that only a few glomerular cells stained positively for CCR2 with a predominant podocyte distribution. In keeping with this, mRNA encoding for CCR2 has been detected by in situ hybridization in normal murine podocytes.29 Glomerular CCR2 expression was evident in kidney biopsies from patients with crescentic GN and localized along the glomerular capillary wall, the Bowman’s capsule, and also in the cellular crescents. Both pattern of staining and colocalization with the podocyte marker synaptopodin provide evidence of CCR2 expression by glomerular podocytes.
Cells positive uniquely for CCR2, either in the glomeruli or in the crescents, are likely to be locally recruited monocytes. However, podocytes migrated into crescents are known to lose specific podocyte markers41; therefore, the possibility that these cells are, at least in part, dedifferentiated podocytes cannot be excluded.
There is evidence that in early crescentic GN, podocytes assume a migratory phenotype and trigger crescent formation by establishing bridges between the tuft and Bowman’s capsule.2,43,44 Furthermore, podocytes undergo proliferation41 and are believed to participate in cellular crescent formation.41,44 Our in vitro data, showing that MCP-1 binding to CCR2 can induce a migratory and proliferative phenotype in podocytes, taken together with our in vivo demonstration of CCR2 expression by podocyte in crescentic GN suggest that MCP-1 can act directly on podocytes and possibly be involved in the podocyte alterations associated with crescent formation. In line with this hypothesis, neutralization of MCP-1 results in a dramatic decrease in glomerular crescent formation.16,45
On the other hand, podocyte migration in response to the MCP-1 may also be implicated in both covering of areas of denudated GBM and foot process broadening and fusion, which requires acquisition of a migratory phenotype. Further studies are required to assess the in vivo relevance of our results showing MCP-1/CCR2-driven podocyte migration.
In conclusion, our data suggest that the MCP-1/CCR2 system is functionally active in podocytes and overexpressed by this cell type in crescentic GN.
Footnotes
Address reprint requests to Dr. Davina Burt, Diabetic Nephropathy Laboratory, Department of Internal Medicine, University of Turin, Turin, 10126, Italy. E-mail: davina.burt@unito.it.
Supported by the European Federation for the Study of Diabetes/Lilly European Diabetes Research Programme and by the Piedmont Region Applied Scientific Research 2004. D.B. is the recipient of a Marie Curie Individual Intra-European Fellowship from the European Community (no. 039574).
References
- Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Le Hir M, Keller C, Eschmann V, Hähnel B, Hosser H, Kriz W. Podocyte bridges between the tuft and Bowman’s capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2001;12:2060–2071. doi: 10.1681/ASN.V12102060. [DOI] [PubMed] [Google Scholar]
- Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol. 2004;15:61–67. doi: 10.1097/01.asn.0000102468.37809.c6. [DOI] [PubMed] [Google Scholar]
- Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- Kakizaki Y, Waga S, Sugimoto K, Tanaka H, Nukii K, Takeya M, Yoshimura T, Yokoyama M. Production of monocyte chemoattractant protein-1 by bovine glomerular endothelial cells. Kidney Int. 1995;48:1866–1874. doi: 10.1038/ki.1995.485. [DOI] [PubMed] [Google Scholar]
- Gruden G, Setti G, Hayward A, Sugden D, Duggan S, Burt D, Buckingham RE, Gnudi L, Viberti G. Mechanical stretch induces monocyte chemoattractant activity via an NF-kappaB-dependent monocyte chemoattractant protein-1-mediated pathway in human mesangial cells: inhibition by rosiglitazone. J Am Soc Nephrol. 2005;16:688–696. doi: 10.1681/ASN.2004030251. [DOI] [PubMed] [Google Scholar]
- Gu L, Hagiwara S, Fan Q, Tanimoto M, Kobata M, Yamashita M, Nishitani T, Gohda T, Ni Z, Qian J, Horikoshi S, Tomino Y. Role of receptor for advanced glycation end-products and signalling events in advanced glycation end-product-induced monocyte chemoattractant protein-1 expression in differentiated mouse podocytes. Nephrol Dial Transplant. 2006;21:299–313. doi: 10.1093/ndt/gfi210. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Chen SF, Zhou H, Chen HP, Li LS. Glomerular expression of C-C chemokines in different types of human crescentic glomerulonephritis. Nephrol Dial Transplant. 2003;18:1526–1534. doi: 10.1093/ndt/gfg172. [DOI] [PubMed] [Google Scholar]
- Prodjosudjadi W, Gerritsma JS, van Es LA, Daha MR, Bruijn JA. Monocyte chemoattractant protein-1 in normal and diseased human kidneys: an immunohistochemical analysis. Clin Nephrol. 1995;44:148–155. [PubMed] [Google Scholar]
- Sassy-Prigent C, Heudes D, Mandet C, Belair MF, Michel O, Perdereau B, Bariety J, Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466–475. doi: 10.2337/diabetes.49.3.466. [DOI] [PubMed] [Google Scholar]
- Eddy AA, Warren JS. Expression and function of monocyte chemoattractant protein-1 in experimental nephrotic syndrome. Clin Immunol Immunopathol. 1996;78:140–151. doi: 10.1006/clin.1996.0023. [DOI] [PubMed] [Google Scholar]
- Hartner A, Veelken R, Wittmann M, Cordasic N, Hilgers KF. Effects of diabetes and hypertension on macrophage infiltration and matrix expansion in the rat kidney. BMC Nephrol. 2005;6:6. doi: 10.1186/1471-2369-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Dorf ME, Proudfoot A, Salant DJ, Gutierrez-Ramos JC. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J Leukoc Biol. 1997;62:676–680. doi: 10.1002/jlb.62.5.676. [DOI] [PubMed] [Google Scholar]
- Wada T, Yokoyama H, Furuichi K, Kobayashi KI, Harada K, Naruto M, Su SB, Akiyama M, Mukaida N, Matsushima K. Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor MCAF/MCP-1. FASEB J. 1996;10:1418–1425. [PubMed] [Google Scholar]
- Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunti S, Pinach S, Arnaldi L, Viberti G, Perin PC, Camussi G, Gruden G. The MCP-1/CCR2 system has direct proinflammatory effects in human mesangial cells. Kidney Int. 2006;69:856–863. doi: 10.1038/sj.ki.5000197. [DOI] [PubMed] [Google Scholar]
- Christensen PJ, Du M, Moore B, Morris S, Toews GB, Paine R., III Expression and functional implications of CCR2 expression on murine alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L68–L72. doi: 10.1152/ajplung.00079.2003. [DOI] [PubMed] [Google Scholar]
- Viedt C, Vogel J, Athanasiou T, Shen W, Orth SR, Kubler W, Kreuzer J. Monocyte Chemoattractant Protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-κB and activator protein-1. Arterioscler Thromb Vasc Biol. 2002;22:914–920. doi: 10.1161/01.atv.0000019009.73586.7f. [DOI] [PubMed] [Google Scholar]
- Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of CCR2 by endothelial cells: implications for MCP-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol. 1999;19:2085–2093. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- Lundien MC, Mohammed KA, Nasreen N, Tepper RS, Hardwick JA, Sanders KL, Van Horn RD, Antony VB. Induction of MCP-1 expression in airway epithelial cells: role of CCR2 receptor in airway epithelial injury. J Clin Immunol. 2002;22:144–152. doi: 10.1023/a:1015420029430. [DOI] [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, Haour F, Parsadaniantz SM. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem. 2002;81:257–269. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Warren GL, Hulderman T, Mishra D, Gao X, Millecchia L, O’Farrell L, Kuziel WA, Simeonova PP. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 2005;19:413–415. doi: 10.1096/fj.04-2421fje. [DOI] [PubMed] [Google Scholar]
- Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- Huber TB, Reinhardt C, Exner M, Burger JA, Kerjaschki D, Saleem MA, Pavenstadt H. Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol. 2002;168:6244–6252. doi: 10.4049/jimmunol.168.12.6244. [DOI] [PubMed] [Google Scholar]
- Rao VH, Meehan DT, Delimont D, Nakajima M, Wada T, Gratton MA, Cosgrove D. Role for macrophage metalloelastase in glomerular basement membrane damage associated with Alport syndrome. Am J Pathol. 2006;169:32–46. doi: 10.2353/ajpath.2006.050896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723–1731. doi: 10.1016/S0002-9440(10)64128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreen N, Mohammed KA, Galffy G, Ward MJ, Antony VB. MCP-1 in pleural injury: cCR2 mediates haptotaxis of pleural mesothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L591–L598. doi: 10.1152/ajplung.2000.278.3.L591. [DOI] [PubMed] [Google Scholar]
- Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- Mirzadegan T, Diehl F, Ebi B, Bhakta S, Polsky I, McCarley D, Mulkins M, Weatherhead GS, Lapierre JM, Dankwardt J, Morgans D, Jr, Wilhelm R, Jarnagin K. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle. J Biol Chem. 2000;275:5562–5571. doi: 10.1074/jbc.M000692200. [DOI] [PubMed] [Google Scholar]
- Stewart RJ, Duley JA, Allardyce RA. The migration of fibroblasts into an in vitro wound. Br J Exp Pathol. 1979;60:582–588. [PMC free article] [PubMed] [Google Scholar]
- Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, Kang SW, Han DS, Han JY, Cha DR. Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol. 2004;82:568–576. doi: 10.1111/j.1440-1711.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- Tylaska LA, Boring L, Weng W, Aiello R, Charo IF, Rollins BJ, Gladue RP. Ccr2 regulates the level of MCP-1/CCL2 in vitro and at inflammatory sites and controls T cell activation in response to alloantigen. Cytokine. 2002;18:184–190. doi: 10.1006/cyto.2002.1031. [DOI] [PubMed] [Google Scholar]
- Luo Y, Dorf ME. Beta-chemokine TCA3 binds to mesangial cells and induces adhesion, chemotaxis, and proliferation. J Immunol. 1996;156:742–748. [PubMed] [Google Scholar]
- Wong LM, Myers SJ, Tsou CL, Gosling J, Arai H, Charo IF. Organization and differential expression of the human monocyte chemoattractant protein 1 receptor gene: evidence for the role of the carboxyl-terminal tail in receptor trafficking. J Biol Chem. 1997;272:1038–1045. doi: 10.1074/jbc.272.2.1038. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int. 2000;58:137–143. doi: 10.1046/j.1523-1755.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- Bariéty J, Bruneval P, Meyrier A, Mandet C, Hill G, Jacquot C. Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int. 2005;68:1109–1119. doi: 10.1111/j.1523-1755.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, de Vries CJ, Egashira K, van Bockel JH, Quax PH. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2063–2069. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- Besse-Eschmann V, Le Hir M, Endlich N, Endlich K. Alteration of podocytes in a murine model of crescentic glomerulonephritis. Histochem Cell Biol. 2004;122:139–149. doi: 10.1007/s00418-004-0683-z. [DOI] [PubMed] [Google Scholar]
- Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]