Abstract
Mesangial cells (MCs) are essential for normal renal function through the synthesis of their own extracellular matrix, which forms the structural support of the renal glomerulus. In many renal diseases this matrix is reorganized in response to a variety of cytokines and growth factors. This study examines proteoglycan and hyaluronan (HA) synthesis by MCs triggered by proinflammatory agents and investigates the effect of an exogenous HA matrix on matrix synthesis by MCs. Metabolic labeling, ion exchange and size exclusion chromatography, Western blotting, and immunocytochemistry were used to identify changes in matrix accumulation. When incubated with interleukin-1, platelet-derived growth factor, or fetal calf serum, MCs initiated rapid HA synthesis associated with the up-regulation of HA synthase-2 and increased the synthesis of versican, perlecan, and decorin/biglycan. HA was both released into the medium and incorporated into extensive pericellular coats. Adding exogenous HA to unstimulated cells that had undetectable pericellular coats of HA selectively reduced perlecan and versican turnover, whereas other proteoglycans were unaffected. These results suggest that high levels of HA in the mesangium in disease is a mechanism controlling the accumulation of specific mesangial matrix components. HA may thus be an attractive target for therapeutic intervention.
The extracellular matrix plays a key role in homeostasis and in maintaining the structural architecture of tissues. In the glomerulus, two separate extracellular matrices have been described, the glomerular basement membrane and the mesangial matrix.1 The glomerular basement membrane forms part of the filtration mechanism, whereas the mesangial matrix forms the structural matrix of the glomerulus.2 The mesangial matrix is synthesized by mesangial cells (MCs) and is a complex mix of glycoproteins such as collagen type IV, laminin, fibronectin, and a number of different proteoglycans.3,4 In the normal kidney these components are maintained at constant levels by the establishment of an equilibrium between their synthesis and degradation.5 In a variety of glomerular diseases, this equilibrium is shifted leading to changes in the amount and composition of the mesangial matrix.6,7,8,9,10,11 This may adversely affect glomerular function leading to glomerulosclerosis and ultimately organ failure. Furthermore, some matrix molecules that have low expression levels or are not present at all in the normal glomerulus may become expressed at high levels in disease. One such molecule is hyaluronan (HA).
HA is a water-soluble, nonsulfated glycosaminoglycan that is a key constituent of the pericellular matrix and has important structural functions in the extracellular matrix of most tissues. It consists of repeating disaccharide units of glucuronic acid and N-acetylglucosamine and is extruded from the plasma membrane as a chain, which may reach a molecular mass of as much as 107 Da. Although in the normal kidney high levels of HA are found only in the renal papilla, there is greatly increased interstitial expression of both HA and its receptor, CD44, in the cortex after acute ischemic injury, interstitial inflammation, or during progressive fibrosis.12,13,14,15 It is an abundant extracellular component of crescents in rat autoimmune glomerulonephritis12,16 and is elevated in proliferating MCs in the Thy-1 model of glomerular injury.17 HA is also expressed at high levels in the glomerulus of rejecting kidneys18,19,20 and in lupus nephritis.15 In vitro MCs synthesize HA in response to low-density lipoprotein,18,19,20,21 fibronectin, or growth factors such as platelet-derived growth factor (PDGF).22 A HA-based matrix is also produced in response to high concentrations of glucose and is assembled by MCs into structures adhesive to monocytes.14,22 The subsequent role of HA in controlling MC function, however, has received little attention. To increase our understanding of the role of HA in affecting MC function, the present study examined the kinetics of HA synthesis by MCs and the subsequent effect of HA on the accumulation of proteoglycans and other matrix constituents.
Materials and Methods
Reagents and Enzymes
Heparinase I, II, and III were purchased from Grampian Enzymes (Orkney, UK). Endotoxin-free sodium hyaluronate preparations of molecular weight 3.9 × 106 and 0.45 × 106 were kind gifts from Dr. Ove Wik (Pharmacia Opthalmics Uppsala, Sweden). Chondroitin ABC lyase and chondroitin CII lyase were from Sigma-Aldrich (Dorset, UK), protease-free chondroitin ABC lyase was from ICN (Thene, UK).
MC Culture and Metabolic Labeling
MCs were established in culture from rat kidney as previously reported by us23,24 and used at passage 5 to 10. They showed positive staining for intracellular myosin and vimentin and were negative for α-smooth muscle actin, cytokeratin, and factor VIII. The growth medium was then removed, the cells washed three times with phosphate-buffered saline (PBS), pH 7.3, and the cells growth-arrested in RPMI 1640 supplemented with 0.2% lactalbumin (LH) for 48 hours. The growth-arrested cells were extensively washed with PBS and metabolically labeled either with 50 μCi/ml carrier-free [35S]-sulfate in sulfate-deficient Dulbecco’s modified Eagle’s medium:RPMI (9:1) or with [6-3H]-glucosamine 20 μCi/ml in RPMI, supplemented with the indicated concentrations of interleukin (IL)-1β, PDGF-BB, or 20% fetal calf serum (FCS) or with 0.2% LH alone as negative control for times up to 36 hours.25,26 The radiolabeled proteoglycans and HA were extracted from the culture medium, the cell layer, or the cell lysate as previously reported in detail by us.24,26
Determination of Cell Growth and DNA Synthesis
Cell numbers were determined indirectly using crystal violet staining. Briefly, the culture medium was removed and the cells washed three times with PBS and incubated with 3% paraformaldehyde for 15 minutes. The fixed cells were then stained with 1% crystal violet in methanol (diluted 1:1 with 3% paraformaldehyde) for 3 minutes, extensively washed with PBS, and air-dried for 15 minutes. The cells were solubilized with 100 μl of 1% sodium dodecyl sulfate overnight at room temperature and the adsorbance at 540 nm determined in a microtiter plate reader (MR 5000/340; Dynatec, Billingshurst, UK). A standard curve revealed a direct correlation between cell number (determined by direct cell counting in an improved Neubauer hemocytometer; Weber Scientific International, Ltd., Teddington, UK) and the adsorbance at 540 nm (r2 = 0.99078) (data not shown).
To determine DNA synthesis, rat MCs were pulsed with 1 μCi/ml [6-3H] thymidine for 24 hours unless otherwise stated. The medium was discarded and the cell layer washed twice with PBS containing 1 mmol/L thymidine before fixing with 500 μl of 5% trichloroacetic acid containing 1 mmol/L thymidine at 4°C for 1 hour. The cell layer was extracted with 500 μl of 0.1 mol/L NaOH at 20°C for 24 hours, aliquots were neutralized with 1 mol/L HCl and the incorporated 3H thymidine determined.
Analytical Gel Chromatography
Gel filtration chromatography of the 35S-labeled proteoglycans was performed after digestion with either chondroitin ABC lyase or heparitinase I, II, and III.24,26 To analyze [3H]-hyaluronan, papain-digested material was divided into two batches. One was digested with hyaluronidase and the other left untreated, and then both were passed over a Sephacryl-500 column as described.26 All profiles shown were generated using extract amounts equivalent to equal numbers of cells.
Selective Digestion of Purified 35S-Labeled Proteoglycans
35S-labeled proteoglycans were incubated either with chondroitin ABC lyase or a combination of heparinases I, II, and III to remove CS/DS chains and HS chains, respectively.27,28
Pulse Chase Experiments
Cells were labeled as before with [35S]-sulfate in the presence of 5 mg/ml of HA for 24 hours. The culture medium was removed and the cells rapidly washed five times with 2 ml pf PBS. The cultures were then chased with RPMI 1640 medium alone or containing 5 mg/ml of HA. At t = 0, 2, 6, 24, and 48 hours the chase medium was decanted and the cell layers differentially extracted as above.
Western Blot Analysis
Purified proteoglycan extracts were digested with either chondroitin ABC lyase or heparinase and the released core proteins subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting using a rabbit polyclonal antiserum raised against cell surface HSPG or a monoclonal antibody (mAb 11B4) against rat perlecan. Rabbit anti-bovine decorin and versican were the kind gift of Prof. D. Heinegard (University of Lund, Lund, Sweden). The anti-HSPG was raised to cell surface HSPG (a mixture of syndecan-1 and syndecan-2) isolated from rat liver membranes, (provided by Dr. Malcolm Lyon, Paterson Institute, Manchester, UK). The mAb 11B4 (a gift from Professor John Couchman, Imperial College, London, UK) was prepared against a HSPG isolated from rat aortic smooth muscle and principally stains all basement membranes of rat tissue. Proteins were visualized using enhanced chemiluminescence (Pharmacia Amersham Biotech, Little Chalfont, UK).
Time Course Experiments
In these experiments rat MCs were grown to 50% confluency and made quiescent as detailed above. At time 0, the experiments were initiated with medium containing either 0.2% lactalbumin or 20% FCS. At T0 and at 4-hour intervals for up to 36 hours cell numbers were determined using the crystal violet method. In parallel experiments rat MC cultures were incubated for 4-hour periods with 3H thymidine for DNA synthesis, [35S]-sulfate for proteoglycan synthesis, and 3H-glucosamine for HA synthesis.
Particle Exclusion Assay
The exclusion of horse erythrocytes was used to visualize the HA pericellular coat. MCs at between 30 and 50% confluence in 35-mm dishes were washed with warm PBS. Formalinized horse erythrocytes (TCS Biosciences Ltd., Botolph Claydon, UK) (500 μl) were added (∼1 × 108/ml) and incubated at 37°C for 15 minutes. Zones of exclusion were visualized and control incubations consisted of cells treated with 12.5 U Streptomyces hyaluronidase for 60 minutes before the addition of erythrocytes.
RNA Extraction and Gene Expression Analysis Using Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
MCs were growth-arrested and then incubated in RPMI 1640 containing either 0.2% LH or 20% FCS for the times shown. Total cellular RNA was extracted with guanidine isothiocyanate followed by centrifugation through 5.7 mol/L CsCl2 in 0.1 mol/L ethylenediaminetetraacetic acid. cDNA was prepared from 1 μg of RNA by reverse transcription at 42°C for 45 minutes using the random hexamer method. PCR amplification was performed in a total volume of 50 μl containing 2 μl of the reverse transcription product, 0.5 μmol/L sense and 0.5 μmol/L antisense primers, 200 μmol/L of dNTPs, 1.25 U of recombinant TaqDNA polymerase in the above PCR buffer. See Jenkins and colleagues26 for details. Densitometric analysis allowed the comparison of each product with the housekeeping gene α-actin.
Immunohistochemistry
Normal (n = 4) and patient (n = 6) biopsy sections were obtained from the pathology archive of the Pathology Department, University Hospital of Wales, with the approval of the local ethics committee. For HA staining, sections were incubated with biotinylated HA-binding protein (b-HABP), at a concentration of 5 μg/ml (Seikagaku Corporation, Tokyo, Japan) at 4°C overnight. The slides were washed with PBS before incubation with fluorescein isothiocyanate (FITC)-avidin-D (20 μg/ml) (Vector Laboratories, Burlingame, CA), at room temperature for 1 hour. Versican was visualized using goat-anti-human versican (Santa Cruz Biotechnology, Santa Cruz, CA) (10 μg/μl) at 4°C overnight and FITC-conjugated rabbit anti-goat IgG secondary antibody. After washing, sections were mounted in Vectashield mounting medium (Vector Laboratories). The numbers of glomeruli visible in each section varied between 5 and 12.
MCs were cultured in LH or FCS on glass coverslips for 24 hours, washed twice with PBS, fixed in ice-cold acetone/methanol (1:1) for 5 minutes, air-dried, and incubated with the relevant antiserum in 1% bovine serum albumin in PBS for 1 hour. The antisera used were all rabbit anti-rat. The cells were washed with 1% bovine serum albumin and incubated with FITC-conjugated goat anti-rabbit IgG (1:80 in PBS, 1% bovine serum albumin) in the dark for 1 hour. The cells were washed with PBS, mounted with Vectashield medium, and examined under a Leitz Orthoplan fluorescent microscope.
Statistics
All results are expressed as mean ± 1 SD. Statistical significance was calculated using the Student’s t-test with a level of significance of P < 0.05.
Results
HA and Versican Expression in the Human Glomerulus
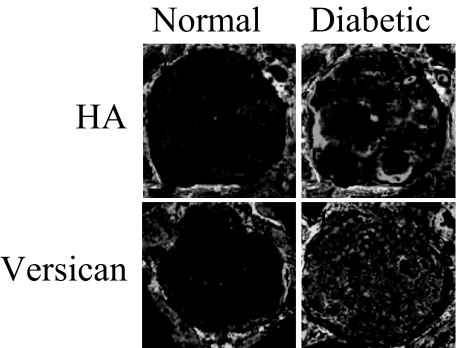
Sections of biopsies from normal volunteers or diabetic patients were stained with either bHABP or anti-versican antibodies. HA was not detected in any of the normal glomeruli examined (Figure 1a), and versican was detected at only low levels (Figure 1c). In contrast, both were visualized in the glomeruli of diabetic patients (Figure 1, b and d).
Figure 1.
Immunohistochemical identification of HA and versican in glomeruli. Sections from normal (a and c) or diabetic (b and d) renal biopsies were immunostained for either HA (a and b) or versican (c and d) as described in Materials and Methods. Glomeruli shown are representative of at least five glomeruli/biopsy of four normal and six patient biopsies examined. Original magnifications, ×250.
Cytokine-Stimulated HA and Proteoglycan Synthesis
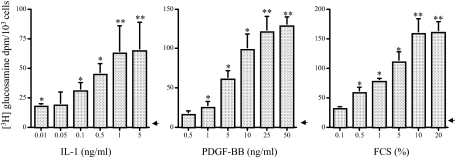
Mesangial cells maintained in a nonproliferative state in medium, containing 0.2% LH, constitutively synthesized low levels of HA (Table 1). This synthesis was dose dependently increased by incubation with IL-1β, PDGF-BB, or concentrations of FCS at 1% or higher (Figure 2). Maximal increases were 13-fold for IL-1β at 5 ng/ml, 25-fold for PDGF-BB at 25 ng/ml, and 32-fold for FCS at 20% throughout a 36-hour incubation period (Table 1). In addition, levels of chondroitinase ABC lyase-degradable CSPG and DSPG also increased to maximal levels at these concentrations of cytokines: IL-1β induced a 2-fold and 1.4-fold increase in CS and DS, respectively, whereas for PDGF-BB the increase was 6.5- and 2.3-fold, respectively. Furthermore, there was also a modest but reproducible rise in HSPG synthesis (1.5-fold with 20% FCS). The identity of the core proteins of the proteoglycans in these samples was established by a combination of size exclusion chromatography (Figure 3) and immunoblotting using well characterized antibodies (Figure 4).
Table 1.
Total Proteoglycan Synthesis by MC
| Conditions | 0.2% LH | IL-1β | PDGF-BB | 20% FCS |
|---|---|---|---|---|
| Hyaluronan* | 5 ± 1 | 65 ± 24§ | 129 ± 11§ | 161 ± 18§ |
| CSPG† | 19 ± 5 | 45 ± 6‡ | 123 ± 37§ | 167 ± 48§ |
| DSPG† | 351 ± 74 | 416 ± 138 | 807 ± 242‡ | 675 ± 178‡ |
| HSPG† | 167 ± 45 | 215 ± 31 | 239 ± 36 | 255 ± 76 |
| Cell numbers (×10−3) | 41 ± 4 | 43 ± 15 | 66 ± 17 | 65 ± 6 |
Cells were metabolically labeled with [35S]-sulfate or [3H]-glucosamine and incubated with the agents shown for 36 hours, as described in Materials and Methods. The conditioned medium and cell extracts were then prepared and specific activity counted as described.
[3H]-glucosamine dpm/103 cells ± 1 SD (n = 3).
[35S]-sulfate dpm/103 cells ± 1 SD (n = 3).
Statistical difference to control levels is shown as
P < 0.05 and
P < 0.01.
Figure 2.
Concentration-dependent HA generation in response to cytokines. Growth-arrested MCs were metabolically labeled with [3H] glucosamine as described in Materials and Methods. They were then incubated with the agents shown for 36 hours. The conditioned medium and cell extracts were then processed and HA-specific activity counted as described. Results are expressed as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with the 0.2% LH control (arrowhead).
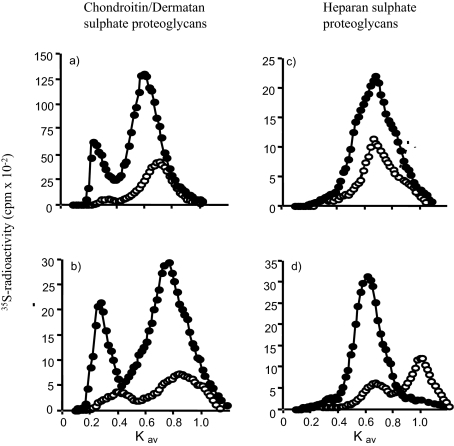
Figure 3.
Sepharose CL-4B elution profiles of 35S-labeled proteoglycans. Growth-arrested MCs were labeled for 24 hours with 50 μCi of [35S]-sulfate in the presence of 0.2% LH (open circles) or 20% FCS (filled circles). Proteoglycans extracted from the culture medium (a and c) and the cell layer (b and d) were digested with heparitinase (a and b) or chondroitin ABC lyase (c and d) and the remaining CSPG and HSPG, respectively, separated on a dissociative Sepharose CL-4B column. Data shown are representative of five independent experiments, each with the initial extracts corrected for equal cell numbers.
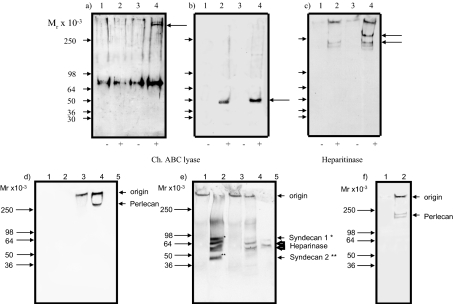
Figure 4.
Western blot analysis of proteoglycans. a–c: Proteoglycans extracted from the culture medium of quiescent (lanes 1 and 2) or proliferating cells (lanes 3 and 4) (a–e) were incubated with buffer alone (lanes 1 and 3) or either chondroitin ABC lyase (a and b, lanes 2 and 4) or heparitinase (c, lanes 2 and 4), then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 3 to 12% gradient gel and Western blotted with rabbit anti-versican (a), rabbit anti-decorin (b), and mAb 11B4 anti-perlecan (c). Mr markers are shown with arrowheads, core proteins with arrows. Note the bands of Mr 68 × 103 in a represent the nonspecific staining of serum albumin by the anti-versican antibody used. d–f: HSPGs were isolated after incubation with chondroitin ABC lyase as described above. Aliquots of the HSPG in the cell lysate (lanes 1 and 2) or matrix (lanes 3 and 4) were incubated with buffer alone (lanes 1 and 3) or with heparinase (lanes 2 and 4) and electrophoresed on 3 to 12% gradient gels after denaturation: without reduction followed by Western blotting with mAb 11B4 (d) and with reduction and blotting with polyclonal anti-cell surface HSPG (e). Lane 5 is heparinase alone. f: Medium HSPG incubated with buffer alone (lane 1) or heparinase (lane 2) and the blot probed with mAb 11B4. Data shown are representative of two independent experiments.
Versican (Figure 4a), decorin (Figure 4b), and perlecan (Figure 4c) were identified, released from the cells. Furthermore, using mAb 11B4, a single band (Mr 300 × 103) was observed in the cytoskeleton matrix extract but not the lysate extract (Figure 4d, compare lane 4 with lane 2). In contrast, the antiserum against the cell surface HSPG revealed four bands after treatment of the cell lysate extract with heparinase, of approximate Mr 90 × 103, 68 × 103, 60 × 103, and 49 × 103 (Figure 4e, lane 2). Based on previous studies with this polyclonal antiserum, it is probable that the 90 × 103 polypeptide is syndecan-1 and the 49 × 103 protein syndecan-2.29,30 The polypeptides at 68 × 103 and 60 × 103 are most likely nonspecific because of cross-reaction with heparinase (Figure 4e, lane 5). The polyclonal antiserum failed to reveal any new bands in the matrix extract (Figure 4e, lanes 3 and 4). These findings suggest that the HSPG peak located in the cytoskeleton matrix contains basement membrane proteoglycans that include perlecan and that the HSPG peak of lower molecular weight is a mixture of cell surface proteoglycans that include syndecan-1 and syndecan-2. Western blot analysis with 11B4 also highlighted two bands at 200 and 180 kDa in the culture medium (Figure 4c). These results taken with the radiolabeling data in Figure 3 suggest that the release of MC layer associated HSPG into the culture medium involves a proteolytic clip of the core protein, a finding that is broadly in agreement with published data in other cell systems.31,32 When the same blot was probed with anti-cell surface HSPG, no protein bands were identified. Based on these observations and previously published biochemical characterization,3,24,33 the majority of the CSPG synthesized was identified as versican, the DSPG was a mixture of both biglycan and decorin, whereas perlecan and the syndecans were the major constituents of the HSPG fraction.
Time Course of Induction of HA and Proteoglycan Synthesis
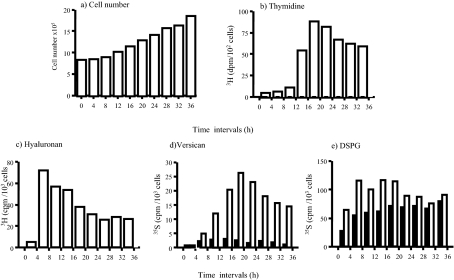
A series of pulse-labeling experiments in which cells were labeled during consecutive 4-hour periods was performed to follow the kinetics of synthesis of HA and the CS/DS PG in quiescent compared with proliferative culture conditions (Figure 5). Conditioned labeling medium was harvested after 4 hours and replaced with fresh medium. This was then harvested and replaced at successive 4-hourly intervals for a total of 36 hours. The levels of all PG and HA synthesized during each period of 4 hours were corrected for cell number. In quiescent cells there was no significant incorporation of [3H]-thymidine or increase in cell number throughout the 36 hours of the experiment (not shown). In proliferating conditions, however, both were significantly increased by 16 hours, with cell number doubling by 36 hours (Figure 5, a and b).
Figure 5.
Kinetics of HA and proteoglycan synthesis. The time course experiments were performed as described in Materials and Methods. a: Cell numbers were determined using the crystal violet staining. b: DNA was determined by the incorporation of 3H thymidine. c: Incorporation of 3H-glucosamine into HA. d: Incorporation of [35S]-sulfate into versican. e: Incorporation of [35S]-sulfate into DSPG. Results are representative of two independent experiments and are expressed as the combined values of cell/matrix-associated and conditioned medium levels of HA and proteoglycans in proliferating (open bars) or nonproliferating (filled bars) cultures. Data shown are for one of two independent experiments that both showed the same patterns of synthesis.
Under nonproliferating conditions, HA synthesis was undetectable during any of the 4-hour time periods (not shown). Similarly, versican synthesis was undetectable after the first 4 hours in nonproliferating conditions, but a low level of synthesis was detectable between 4 and 8 hours, remaining low in each 4-hour period for the full 36 hours of the experiment (Figure 5d, black columns). DS synthesis was fairly constant during each period of 4 hours throughout the chase period (Figure 3e, black columns). In contrast, under proliferating conditions, the synthesis of HA and versican was markedly increased above that of quiescent cells (Figure 5, c and d; open columns). The synthesis of HA reached a maximum in the 4-hour chase period between 4 and 8 hours and declined in each subsequent 4-hour period to ∼30% of its maximum during the 20- to 24-hour period. In comparison, versican synthesis peaked at a later time than HA, in the period between 16 and 24 hours, coinciding with the peak of DNA synthesis (Figure 5b). Thereafter, although remaining considerably greater than that of quiescent cells, versican generation declined. DSPG synthesis was also increased in proliferative conditions, to a maximum of twofold between 8 and 12 hours (Figure 5e, open columns), but returned to quiescent levels by 20 to 24 hours.
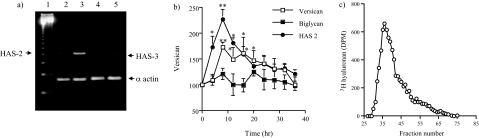
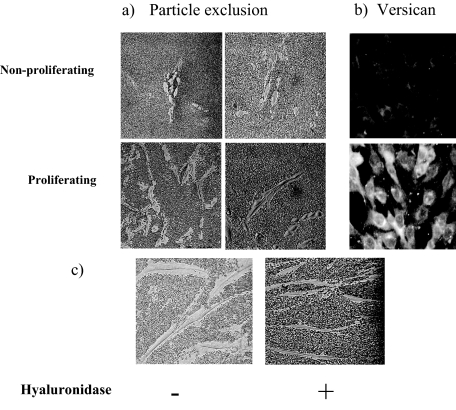
The striking finding from this series of experiments was the rapid induction of HA synthesis that had been undetectable in quiescent cells. This was shown by semiquantitative RT-PCR to be the result of an increase in the steady state levels of the mRNA for the HAS2 synthase enzyme as the levels for HAS-3 did not change (Figure 6a) and HAS-1 was undetectable in these cells. Furthermore, RT-PCR of mRNA extracted at 4-hour intervals demonstrated that the increase in the level of the mRNA for HAS-2 preceded that of versican and that the mRNA for the DSPG biglycan stayed comparatively static (Figure 6b). In addition, size exclusion chromatography demonstrated that the HA synthesized was predominantly of a high-molecular weight (750 to 2000 kDa) (Figure 6c). Similarly there was an increase in cell-associated HA, reflected in the size of the pericellular HA coat visualized by particle exclusion assay. The pericellular coat of quiescent cells was undetectable whereas that of proliferating MCs was extensive, leaving a clear exclusion zone around each cell (Figure 7a). In addition, immunohistochemistry indicated that in proliferating cells there was a marked increase in the levels of pericellular versican (Figure 7b), suggesting that the HA and versican that are induced in proliferating conditions are rapidly incorporated into a reorganized HA-rich pericellular matrix.
Figure 6.
Versican, biglycan, and HAS-2 mRNA expression and HA synthesis. a: MCs were cultured in LH (lanes 2 and 4) or 20% FCS (lanes 3 and 5) and mRNA extracted, reverse-transcribed, and HAS-2 and HAS-3 PCR-amplified using α-actin as the housekeeping gene. Lane 1: DNA ladder markers. PCR products of HAS-2 (lanes 2 and 3) and HAS-3 (lanes 4 and 5). Results are representative of three independent experiments. b: mRNA was extracted at the times shown from cells induced to proliferate. Versican, biglycan, and HAS-2 were then PCR-amplified and the products separated on 3% agarose gels. Densitometric analysis was then performed and the values normalized using α-actin as the housekeeping gene. The results are the means ± SD for three independent experiments. *P < 0.05, **P < 0.01 compared with time 0. c: Proliferating MCs were radiolabeled with [6-3H]-glucosamine as described and the HA in the conditioned medium eluted from a Sepharose S500 column. Results are representative of six independent analyses.
Figure 7.
Visualization of the HA pericellular coat. MCs in nonproliferating (0.2% LH) or proliferating (20% FCS) conditions were incubated with formalized erythrocytes for the particle exclusion assay (a) or incubated with anti-versican antibody and visualized with anti-rabbit-FITC IgG (b). c: Loss of pericellular coat in proliferating cells incubated with hyaluronidase as a control. Data shown are representative of five independent experiments. Original magnifications: ×200 (a); ×250 (b); ×400 (c).
The Effect of HA on MC Extracellular Matrix Synthesis
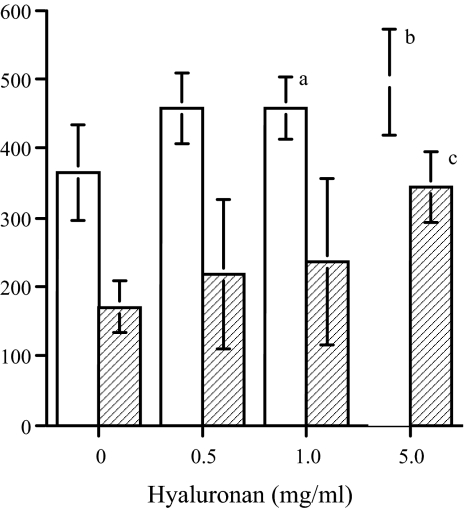
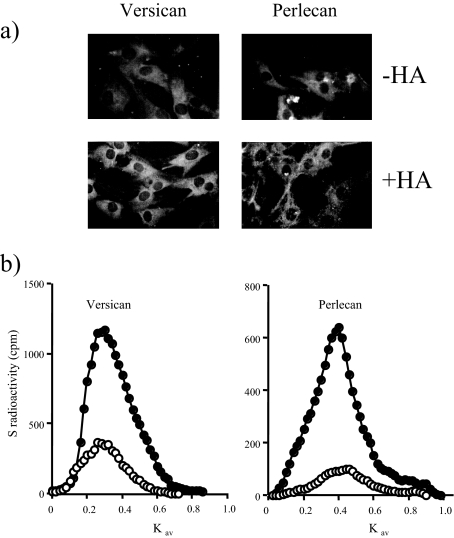
To examine whether an increase in HA could directly influence MC matrix synthesis in the absence of any other stimulus, quiescent cells were incubated in the presence of HA, applied exogenously. Methyl cellulose (Sigma-Aldrich) was used as a negative control to give a comparable zero shear viscosity to the highest concentration of HA used (∼100 Pa.s.). Exogenous HA induced a small but significant concentration-dependent increase in the accumulation of extractable [35S]-labeled proteoglycans in the conditioned medium and cell/matrix layer throughout a 24-hour period (Figure 8). The increase in the cell layer, however, was greater as a proportion of the untreated levels than that in the medium, and methyl cellulose was without effect (Table 2). Immunohistochemistry (Figure 9a) and analysis of the cell layer extracts by specific enzymatic digestion and size exclusion chromatography (Figure 9b) showed that this was explained by selective threefold and ninefold increases in versican and perlecan, respectively. There was little difference in the accumulation of DSPG (not shown) and no significant effect on proliferation or viability in any of the experiments (Table 2).
Figure 8.
The effect of HA on the incorporation of [35S]-sulfate into proteoglycans. MCs were labeled for 24 hours with [35S]-sulfate in the presence of increasing concentrations of HA. The culture medium (open bars) and the cell layer (shaded bars) were extracted and the 35S-labeled proteoglycans quantitated using DEAE ion exchange chromatography as described in Materials and Methods. The results are expressed as 35S-proteoglycans (cpm/103 cells ± 1 SD): aP < 0.5, bP < 0.01, cP < 0.001 versus medium alone, n = 8.
Table 2.
Total Proteoglycan Accumulation after HA Addition
| Hyaluronan | Conditioned medium
|
Cell layer
|
Cell number (×10−3) | ||
|---|---|---|---|---|---|
| HSPG | CS/DS PG | HSPG | CS/DS PG | ||
| None | 84 ± 26 | 281 ± 58 | 84 ± 20 | 87 ± 22 | 46 ± 7 |
| LMW (5 mg/ml) | 133 ± 50 | 359 ± 68 | 165 ± 43* | 110 ± 17* | 31 ± 12 |
| HMW (5 mg/ml) | 136 ± 16 | 361 ± 70 | 188 ± 35† | 156 ± 18† | 39 ± 9 |
| Methyl cellulose (1.5%) | 61 ± 19 | 223 ± 81 | 93 ± 27 | 98 ± 16 | 43 ± 8 |
Cells were incubated with or without HA or methyl cellulose and metabolically labeled with [35S]-sulfate as described in Materials and Methods. Culture media were collected and cell layers extracted and purified using DEAE chromatography. Aliquots were then digested with chondroitin ABC-lyase or heparitinase and analyzed on Sepharose CL 4B columns. Results are expressed as 35S-sulfate cpm/103cells ± 1 SD (n = 5 for LMW and HMW HA and n = 3 for methyl cellulose).
P < 0.05,
P < 0.01 compared to 0.2% LH.
Figure 9.
The effect of HA on cell-associated versican and perlecan expression. a: MCs were incubated in the presence or absence of 5 mg/ml of HA and then fixed and immunostained with anti-versican or anti-perlecan antibodies and visualized with anti-rabbit FITC-conjugated IgG. b: MCs were radiolabeled for 24 hours with [35S]sulfate in the presence (filled circles) or absence (open circles) of 5 mg/ml of HA. Cell layer proteoglycans were then quantified using DEAE ion exchange and gel exclusion chromatography as described in Materials and Methods. The results are expressed as 35S-proteoglycans (cpm/103 cells). Data shown are representative of three independent experiments.
Pulse Chase Experiments
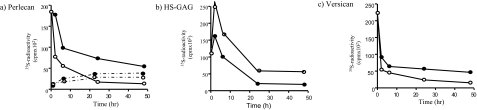
Pulse-chase experiments were performed to investigate whether these increases were attributable to an alteration in the metabolic fate of the cell-associated PGs. Cells were labeled with [35S]-sulfate in the presence of HA for 24 hours and then chased in isotope-free medium containing either HA or culture medium alone. At defined times the conditioned medium was collected, the cell layer differentially extracted and each fraction separately chromatographed on Sepharose CL-4B after digestion with either chondroitin ABC lyase or heparinase. Overall the decay of HSPG from the cell layer was not matched by the appearance of an equal amount in the chase medium (Figure 10a). This suggested that some HSPG was lost from the culture, probably by internalization and complete digestion by the lysosomal system. This amounted to 58% under control conditions and 49% in the presence of added HA. Therefore the presence of HA appeared to slow down the overall turnover of HSPG. Analysis of the matrix and cell-associated HSPG showed that this effect was accounted for by changes in the metabolism of perlecan. Furthermore, in the presence of HA there was a decrease in the intracellular 35S-HS-GAG fraction (Figure 10b), indicating that less of the matrix HSPG entered the metabolic pool.
Figure 10.
The effect of HA on the turnover of perlecan and versican. MCs were pulse-labeled for 24 hours in the presence of HA (5 mg/ml) and chased in either medium alone (open circles) or medium containing HA (5 mg/ml) (filled circles) for periods up to 48 hours. At the time points indicated the chase medium was decanted, the cell layer differentially extracted, and the cpm in the peaks corresponding to either perlecan (a and b) or versican (c) quantitated after digestion with heparinase or chondroitinase ABC, respectively, as described in Materials and Methods. The intracellular HS GAG levels are shown in b. Solid lines represent counts remaining cell-associated, and dashed lines are counts released to the medium. Results are representative of three independent experiments.
Analysis of versican showed that in control cultures the majority was rapidly lost from the cell layer (Figure 10c). Because this proteoglycan was not detected in the cell medium it was probably removed from the system via internalization and complete degradation. As with perlecan the inclusion of HA in the chase medium served to slow down this metabolism.
To exclude the possibility that the observed results were attributable to nonspecific trapping of the larger proteoglycans by the high-molecular weight HA, the effect of an HA preparation of small hydrodynamic size (Mr ∼450 kDa) was examined. This preparation, at the same concentration, induced each proteoglycan species to a similar degree as that of the larger molecule, although the induction of cell layer versican was only ∼50% of that of the higher molecular weight molecule (Table 2). Furthermore, using methyl cellulose as a viscosity control did not reproduce the effects induced by high-molecular weight HA (Table 2). This suggested that the observed effects were attributable to specific interactions with HA rather than to nonspecific trapping of the proteoglycans. There was no change in proteoglycan synthesis by the cells in response to oligosaccharides generated by the digestion of HA by Streptomyces sp. hyaluronidase (not shown).
Discussion
HA and versican-rich glomerular matrices were observed in biopsy sections of diabetic kidneys, although neither was present in normal glomeruli. Furthermore, both have been reported to accumulate in several other glomerular diseases.15,19,20,34 However, the rapid initiation of HA synthesis and the greatly increased synthesis of versican by MCs in response to the mediators used in this report have not been demonstrated previously. The MC response to glomerular injury has been attributed to multiple factors, including cytokines/growth factors, hormones, inflammatory products, and lipids.7,35,36,37 Many of these molecules have mitogenic activity for cultured MCs (eg, PDGF). Furthermore, some have been demonstrated to increase the rate of biosynthesis of matrix components associated with the pathological mesangium. The growth factor PDGF, for example, has been highlighted both in vitro and in vivo as a particularly important mediator of mesangial reorganization, whereas IL-1 is a major cytokine implicated in inflammatory glomerular disease.36,37,38 Although these mediators induce different responses from MCs, this is the first report to examine and compare their effect on glycosaminoglycan synthesis. For these reasons we used both PDGF and IL-1 in our experiments with 0.2% lactalbumin hydrolysate (LH) as a nonproliferative medium and FCS as a proliferative stimulus to which they could be compared. Both LH and FCS incubations are conditions that we have used previously in extensive studies on the synthesis of matrix constituents and proteinases by MCs39,40,41,42,43 and thus represented well established control conditions.
In scarring and wound healing, there is a well defined sequence of matrix induction in which HA synthesis is a very early feature. This is under the control of a range of mediators including profibrotic cytokines and growth factors. HA synthesis is rarely induced in isolation, however, and the formation of HA-versican matrices is a major mechanism controlling cell function and an important contributory factor to disease.44 HA binds to the amino terminal G1 domain of versican and this binding together with that of link protein is essential for the stability of the HA-rich matrix. Indeed the HA-versican complex has been described as an important mechanism controlling cell shape and division, without which arterial smooth muscle cells, for example, do not proliferate.44 The present report confirms the importance of the HA matrix but also demonstrates that the addition of HA to MCs selectively alters the accumulation of matrix components including versican and the large heparan sulfate proteoglycan perlecan.
HA synthesis occurs at the plasma membrane through the action of one or more of three HA synthase enzymes.45,46,47 These enzymes orchestrate the polymerization of HA and its translocation to the pericellular and extracellular matrix. They each have distinct tissue and temporal expression that dictates their role in regulating HA biosynthesis. The transcriptional regulation of these enzymes, however, has only recently been addressed,48,49,50 and it is not clear whether agents that induce one isoform also induce the others or at what level this is controlled. Nevertheless, in the present study it was clear that the cytokine-induced initiation of HA synthesis was the result of a rapid, specific, and independent induction of only HAS-2 mRNA, similar to that reported in mesothelial cells,51 proximal tubular epithelial cells,52 and in lung fibroblasts.53 These effects occurred independently of proliferation because IL-1β was not mitogenic for MCs yet it was a strong inducer of both HA and versican. In the absence of any stimulus, MCs did not synthesize HA and synthesized only low basal levels of versican.
To investigate the effect of HA itself on MC matrix production, cells were incubated in the presence of increasing concentrations of high-molecular weight or low-molecular weight HA or HA oligosaccharides. There was a significant increase in the amount of versican and the large HSPG perlecan in the cell layer in a concentration-dependent manner to both preparations of intact HA but not to the fully digested oligosaccharides, which were too short to bind to the HA receptor CD44. Previous studies from several laboratories suggest that HA may influence cell proliferation but that this is dependent on the cell line under investigation and on the dose and molecular weight of the HA.54,55,56,57,58 In our studies, exogenous HA in the concentration range of 0.1 to 5 mg/ml had no significant effect on the proliferation of MCs when determined by 3H-thymidine incorporation or by direct cell counting. Moreover, HA had no adverse effect on cell viability. Thus, the overall increase in the level of 35S-labeled proteoglycan was not accounted for by a proliferative response of the cells to exogenous HA. This is particularly important for the interpretation of the data with regards to the selective increase in HSPG and CSPG because it suggests that HA can specifically influence the in vitro accumulation of both of these proteoglycans by MCs, independently of cell proliferation.
There are two potential mechanisms that would explain these results that are not mutually exclusive. First, HA, through the formation of hydrophobic bonds, self-associates to form a meshwork.59 The exact form of this will depend on the molecular mass and concentration of HA. Alternatively, the HA could form a scaffold on which other molecules can interact to establish a diffusion barrier. A number of proteins, glycoproteins, and proteoglycans bind to, or interact with, HA. These include HA-binding proteins such as cartilage link protein, TSG-6, chondroitin sulfate proteoglycans such as versican and brevican, and the HA membrane receptor CD44.60 All these molecules have a link module that interacts with HA. In addition, inter-α-trypsin inhibitor, collagen VI, and fibronectin also interact with HA to form complexes. These interactions with HA play a critical role in the formation and stability of the extracellular matrix as well as regulating certain aspects of cell behavior. We favor this interpretation because the observed effects were reproduced by using HA of a lower molecular weight, which would not form meshwork structures at the same concentrations.
Although HA is not found in the normal renal cortical interstitium, it is an abundant component of glomerular crescents and of the fibrotic interstitium observed in a number of different renal diseases. It is still not clear whether its presence is indicative simply of tissue undergoing reorganization and repair or whether it contributes directly to the fibrotic processes that lead to organ failure. Recent studies suggest that it is the extracellular, macromolecular packaging of HA into pericellular coats or into cable-like structures that may partly determine its role.14,61,62 Although we did not look specifically for cables of HA, pericellular HA coats were observed around MCs that had been induced to synthesize HA. When exogenous HA was added as an approximation of an extracellular HA matrix, there was a direct effect on the synthesis of and incorporation of both versican and perlecan.
Changes in the heparan sulfate content of glomeruli in experimental models of diabetes have been described recently.63 These changes coincide with the accumulation of HA described by others.54,64 Furthermore, the heparan sulfate chains were not cell surface,63 indicating that they were expressed on large proteoglycans such as perlecan or agrin. Our findings would suggest, therefore, that it is the accumulation of HA that mediates changes in glomerular proteoglycans. Furthermore, because there appeared to be differences in the proteoglycan profiles stimulated by FCS and those accumulated by HA addition, there may also be important distinctions in the way the cell perceives endogenously produced HA and HA added exogenously. We are currently examining this possibility. We have also recently examined the differences in cell function dependent on the overexpression of either HAS-2 or HAS-3. Although HAS-2 expression was associated with pericellular coat formation, HAS-3 overexpression induced the formation of HA cables.61,62 The involvement of HA-binding proteins and matrix constituents in these phenotypes is currently being investigated. These findings are similar to those reported recently for myofibroblasts differentiated from fibroblasts under the influence of transforming growth factor β1, in which the differentiation and change in matrix synthesis are directly related to HA accumulation.26 Taken together these results suggest that changes in HA synthesis induced in a variety of cell types may directly affect cell function and may represent one of the factors influencing whether there is resolution or progression of disease.
Acknowledgments
We thank Dr. Saphwan Al-Assaf, North-East Wales Institute, Wrexham, UK, for advice on viscosity measurements of HA and methyl cellulose.
Footnotes
Address reprint requests to Robert Steadman, Institute of Nephrology, Cardiff University, Heath Park, Cardiff, CF14 4XN, UK. E-mail: steadmanr@cf.ac.uk.
Supported by the Kidney Wales Foundation, Kidney Research UK, and the Deutsche Forschungsgemeinschaft (postdoctoral fellowship KA 1105/1-1 and -2 to S.K.).
References
- Sterzel RB. Interaction of mesangial cells and the extracellular matrix in the glomerulus. Verh Dtsch Ges Pathol. 1989;73:36–38. [PubMed] [Google Scholar]
- Sterzel RB, Hartner A, Hilgers KF, Bressan GM. Contribution of the mesangium to elastic strength and anchorage of the glomerular capillary tuft. Contrib Nephrol. 2001;131:132–141. doi: 10.1159/000060070. [DOI] [PubMed] [Google Scholar]
- Davies M, Thomas GJ, Shewring LD, Mason RM. Mesangial cell proteoglycans: synthesis and metabolism. J Am Soc Nephrol. 1992;2:S88–S94. doi: 10.1681/ASN.V210s88. [DOI] [PubMed] [Google Scholar]
- Rupprecht HD, Schocklmann HO, Sterzel RB. Cell-matrix interactions in the glomerular mesangium. Kidney Int. 1996;49:1575–1582. doi: 10.1038/ki.1996.228. [DOI] [PubMed] [Google Scholar]
- Davies M, Coles GA, Thomas GJ, Martin J, Lovett DH. Proteinases and the glomerulus: their role in glomerular diseases. Klin Wochenschr. 1990;68:1145–1149. doi: 10.1007/BF01798066. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Makino H, Ota Z. Alterations in glomerular extracellular matrix components in glomerulonephritis. Nippon Jinzo Gakkai Shi. 1993;35:941–947. [PubMed] [Google Scholar]
- Schoecklmann HO, Rupprecht HD, Gauer S, Yao J, Sterzel RB. Mesangial cell-matrix interactions in glomerular inflammation. Kidney Blood Press Res. 1996;19:184–190. doi: 10.1159/000174071. [DOI] [PubMed] [Google Scholar]
- Harendza S, Schneider A, Helmchen U, Stahl RA. Extracellular matrix deposition and cell proliferation in a model of chronic glomerulonephritis in the rat. Nephrol Dial Transplant. 1999;14:2873–2879. doi: 10.1093/ndt/14.12.2873. [DOI] [PubMed] [Google Scholar]
- Cybulsky AV. Growth factor pathways in proliferative glomerulonephritis. Curr Opin Nephrol Hypertens. 2000;9:217–223. doi: 10.1097/00041552-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Raptis AE, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S424–S437. doi: 10.1055/s-2001-18600. [DOI] [PubMed] [Google Scholar]
- Fornoni A, Striker LJ, Zheng F, Striker GE. Reversibility of glucose-induced changes in mesangial cell extracellular matrix depends on the genetic background. Diabetes. 2002;51:499–505. doi: 10.2337/diabetes.51.2.499. [DOI] [PubMed] [Google Scholar]
- Jun Z, Hill PA, Lan HY, Foti R, Mu W, Atkins RC, Nikolic-Paterson DJ. CD44 and hyaluronan expression in the development of experimental crescentic glomerulonephritis. Clin Exp Immunol. 1997;108:69–77. doi: 10.1046/j.1365-2249.1997.d01-977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Babazono T, Nitta K, Iwamoto Y. High glucose stimulates hyaluronan production by renal interstitial fibroblasts through the protein kinase C and transforming growth factor-beta cascade. Metabolism. 2001;50:789–794. doi: 10.1053/meta.2001.24207. [DOI] [PubMed] [Google Scholar]
- Wang A, Hascall VC. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J Biol Chem. 2004;279:10279–10285. doi: 10.1074/jbc.M312045200. [DOI] [PubMed] [Google Scholar]
- Yung S, Tsang RC, Leung JK, Chan TM. Increased mesangial cell hyaluronan expression in lupus nephritis is mediated by anti-DNA antibody-induced IL-1beta. Kidney Int. 2006;69:272–280. doi: 10.1038/sj.ki.5000042. [DOI] [PubMed] [Google Scholar]
- Hallgren R, Gerdin B, Tufveson G. Hyaluronic acid accumulation and redistribution in rejecting rat kidney graft. Relationship to the transplantation edema. J Exp Med. 1990;171:2063–2076. doi: 10.1084/jem.171.6.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic-Paterson DJ, Jun Z, Tesch GH, Lan HY, Foti R, Atkins RC. De novo CD44 expression by proliferating mesangial cells in rat anti-Thy-1 nephritis. J Am Soc Nephrol. 1996;7:1006–1014. doi: 10.1681/ASN.V771006. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Andres G, Bhan AK, McCluskey RT, Collins AB, Stow JL, Stamenkovic I. Hyaluronate is a component of crescents in rat autoimmune glomerulonephritis. Lab Invest. 1993;68:146–153. [PubMed] [Google Scholar]
- Wells A, Larsson E, Hanas E, Laurent T, Hallgren R, Tufveson G. Increased hyaluronan in acutely rejecting human kidney grafts. Transplantation. 1993;55:1346–1349. doi: 10.1097/00007890-199306000-00025. [DOI] [PubMed] [Google Scholar]
- Wells A, Larsson E, Fellstrom B, Tufveson G, Klareskog L, Laurent T. Role of hyaluronan in chronic and acutely rejecting kidneys. Transplant Proc. 1993;25:2048–2049. [PubMed] [Google Scholar]
- Chana RS, Wheeler DC, Thomas GJ, Williams JD, Davies M. Low-density lipoprotein stimulates mesangial cell proteoglycan and hyaluronan synthesis. Nephrol Dial Transplant. 2000;15:167–172. doi: 10.1093/ndt/15.2.167. [DOI] [PubMed] [Google Scholar]
- Dunlop ME, Clark S, Mahadevan P, Muggli E, Larkins RG. Production of hyaluronan by glomerular mesangial cells in response to fibronectin and platelet-derived growth factor. Kidney Int. 1996;50:40–44. doi: 10.1038/ki.1996.284. [DOI] [PubMed] [Google Scholar]
- Martin J, Lovett DH, Gemsa D, Sterzel RB, Davies M. Enhancement of glomerular mesangial cell neutral proteinase secretion by macrophages: role of interleukin 1. J Immunol. 1986;137:525–529. [PubMed] [Google Scholar]
- Thomas GJ, Shewring L, McCarthy KJ, Couchman JR, Mason RM, Davies M. Rat mesangial cells in vitro synthesize a spectrum of proteoglycan species including those of the basement membrane and interstitium. Kidney Int. 1995;48:1278–1289. doi: 10.1038/ki.1995.412. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Jenner L, Mason RM, Davies M. Human glomerular epithelial cell proteoglycans. Arch Biochem Biophys. 1990;278:11–20. doi: 10.1016/0003-9861(90)90224-m. [DOI] [PubMed] [Google Scholar]
- Jenkins RH, Thomas GJ, Williams JD, Steadman R. Myofibroblastic differentiation leads to hyaluronan accumulation through reduced hyaluronan turnover. J Biol Chem. 2004;279:41453–41460. doi: 10.1074/jbc.M401678200. [DOI] [PubMed] [Google Scholar]
- Clayton A, Thomas J, Thomas GJ, Davies M, Steadman R. Cell surface heparan sulfate proteoglycans control the response of renal interstitial fibroblasts to fibroblast growth factor-2. Kidney Int. 2001;59:2084–2094. doi: 10.1046/j.1523-1755.2001.00723.x. [DOI] [PubMed] [Google Scholar]
- Thomas G, Clayton A, Thomas J, Davies M, Steadman R. Structural and functional changes in heparan sulfate proteoglycan expression associated with the myofibroblastic phenotype. Am J Pathol. 2003;162:977–989. doi: 10.1016/S0002-9440(10)63892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A, Lyon M, Hampson IN, Cowling GJ, Gallagher JT. Molecular cloning of the major cell surface heparan sulfate proteoglycan from rat liver. J Biol Chem. 1992;267:3894–3900. [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell. 1994;5:183–192. doi: 10.1091/mbc.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter SR, Tyree B, Hassell JR, Horigan EA. Identification of the precursor protein to basement membrane heparan sulfate proteoglycans. J Biol Chem. 1985;260:8106–8113. [PubMed] [Google Scholar]
- Heremans A, van der Schueren B, de Cock B, Paulsson M, Cassiman JJ, van den Berghe H, David G. Matrix-associated heparan sulfate proteoglycan: core protein-specific monoclonal antibodies decorate the pericellular matrix of connective tissue cells and the stromal side of basement membranes. J Cell Biol. 1989;109:3199–3211. doi: 10.1083/jcb.109.6.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. Glomerular cell proteoglycans: their possible role in progressive glomerular disease. Exp Nephrol. 1995;3:122–126. [PubMed] [Google Scholar]
- Stokes MB, Hudkins KL, Zaharia V, Taneda S, Alpers CE. Up-regulation of extracellular matrix proteoglycans and collagen type I in human crescentic glomerulonephritis. Kidney Int. 2001;59:532–542. doi: 10.1046/j.1523-1755.2001.059002532.x. [DOI] [PubMed] [Google Scholar]
- Schöcklmann HO, Lang S, Sterzel RB. Regulation of mesangial cell proliferation. Kidney Int. 1999;56:1199–1207. doi: 10.1046/j.1523-1755.1999.00710.x. [DOI] [PubMed] [Google Scholar]
- Ostendorf T, Burg M, Floege J. Cytokines and glomerular injury. Kidney Blood Pres Res. 1996;19:281–289. doi: 10.1159/000174088. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Fine LG. The concept of glomerular self-defense. Kidney Int. 1999;55:1639–1671. doi: 10.1046/j.1523-1755.1999.00425.x. [DOI] [PubMed] [Google Scholar]
- Floege J, Ostendorf T. Platelet-derived growth factor: a new clinical target on the horizon. Kidney Int. 2001;59:1592–1593. doi: 10.1046/j.1523-1755.2001.0590041592.x. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Mason RM, Davies M. Characterization of proteoglycans synthesized by human adult glomerular mesangial cells in culture. Biochem J. 1991;277:81–88. doi: 10.1042/bj2770081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GJ, Bayliss MT, Harper K, Mason RM, Davies M. Glomerular mesangial cells in vitro synthesize an aggregating proteoglycan immunologically related to versican. Biochem J. 1994;302:49–56. doi: 10.1042/bj3020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Knowlden J, Davies M, Williams JD. Identification and independent regulation of human mesangial cell metalloproteinases. Kidney Int. 1994;46:877–885. doi: 10.1038/ki.1994.345. [DOI] [PubMed] [Google Scholar]
- Martin J, Eynstone L, Davies M, Steadman R. Induction of metalloproteinases by glomerular mesangial cells stimulated by proteins of the extracellular matrix. J Am Soc Nephrol. 2001;12:88–96. doi: 10.1681/ASN.V12188. [DOI] [PubMed] [Google Scholar]
- Martin J, Eynstone LV, Davies M, Williams JD, Steadman R. The role of ADAM 15 in glomerular mesangial cell migration. J Biol Chem. 2002;277:33683–33689. doi: 10.1074/jbc.M200988200. [DOI] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Itano N, Kimata K. Molecular cloning of human hyaluronan synthase. Biochem Biophys Res Commun. 1996;222:816–820. doi: 10.1006/bbrc.1996.0827. [DOI] [PubMed] [Google Scholar]
- Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- Itano N, Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002;54:195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- Monslow J, Williams JD, Norton N, Guy CA, Price IK, Coleman SL, Williams NM, Buckland PR, Spicer AP, Topley N, Davies M, Bowen T. The human hyaluronan synthase genes: genomic structures, proximal promoters and polymorphic microsatellite markers. Int J Biochem Cell Biol. 2003;35:1272–1283. doi: 10.1016/s1357-2725(03)00048-7. [DOI] [PubMed] [Google Scholar]
- Monslow J, Williams JD, Guy CA, Price IK, Craig KJ, Williams HJ, Williams NM, Martin J, Coleman SL, Topley N, Spicer AP, Buckland PR, Davies M, Bowen T. Identification and analysis of the promoter region of the human hyaluronan synthase 2 gene. J Biol Chem. 2004;279:20576–20581. doi: 10.1074/jbc.M312666200. [DOI] [PubMed] [Google Scholar]
- Monslow J, Williams JD, Fraser DJ, Michael DR, Foka P, Kift-Morgan AP, Luo DD, Fielding CA, Craig KJ, Topley N, Jones SA, Ramji DP, Bowen T. Sp1 and Sp3 mediate constitutive transcription of the human hyaluronan synthase 2 gene. J Biol Chem. 2006;281:18043–18050. doi: 10.1074/jbc.M510467200. [DOI] [PubMed] [Google Scholar]
- Yung S, Coles GA, Davies M. IL-1 beta, a major stimulator of hyaluronan synthesis in vitro of human peritoneal mesothelial cells: relevance to peritonitis in CAPD. Kidney Int. 1996;50:1337–1343. doi: 10.1038/ki.1996.446. [DOI] [PubMed] [Google Scholar]
- Jones S, Phillips AO. Regulation of renal proximal tubular epithelial cell hyaluronan generation: implications for diabetic nephropathy. Kidney Int. 2001;59:1739–1749. doi: 10.1046/j.1523-1755.2001.0590051739.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson TS, Potter-Perigo S, Tsoi C, Altman LC, Wight TN. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am J Respir Cell Mol Biol. 2004;31:92–99. doi: 10.1165/rcmb.2003-0380OC. [DOI] [PubMed] [Google Scholar]
- Mahadevan P, Larkins RG, Fraser JR, Dunlop ME. Effect of prostaglandin E2 and hyaluronan on mesangial cell proliferation. A potential contribution to glomerular hypercellularity in diabetes. Diabetes. 1996;45:44–50. doi: 10.2337/diab.45.1.44. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Sakuta T, Yamaguchi T. Effects of hyaluronan on cell proliferation and proteoglycan synthesis in rabbit ligamental cells. Int J Tissue React. 1996;18:87–95. [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Nasreen N, Mohammed KA, Hardwick J, Van Horn RD, Sanders K, Kathuria H, Loghmani F, Antony VB. Low molecular weight hyaluronan induces malignant mesothelioma cell (MMC) proliferation and haptotaxis: role of CD44 receptor in MMC proliferation and haptotaxis. Oncol Res. 2002;13:71–78. [PubMed] [Google Scholar]
- Nilsson SK, Haylock DN, Johnston HM, Occhiodoro T, Brown TJ, Simmons PJ. Hyaluronan is synthesized by primitive hemopoietic cells, participates in their lodgment at the endosteum following transplantation, and is involved in the regulation of their proliferation and differentiation in vitro. Blood. 2003;101:856–862. doi: 10.1182/blood-2002-05-1344. [DOI] [PubMed] [Google Scholar]
- Scott JE, Cummings C, Brass A, Chen Y. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem J. 1991;274:699–705. doi: 10.1042/bj2740699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277:4585–4588. doi: 10.1074/jbc.R100036200. [DOI] [PubMed] [Google Scholar]
- Selbi W, Day AJ, Rugg MS, Fulop C, de la Motte CA, Bowen T, Hascall VC, Phillips AO. Overexpression of hyaluronan synthase 2 alters hyaluronan distribution and function in proximal tubular epithelial cells. J Am Soc Nephrol. 2006;17:1553–1567. doi: 10.1681/ASN.2005080879. [DOI] [PubMed] [Google Scholar]
- Selbi W, de la Motte CA, Hascall VC, Day AJ, Bowen T, Phillips AO. Characterization of hyaluronan cable structure and function in renal proximal tubular epithelial cells. Kidney Int. 2006;70:1287–1295. doi: 10.1038/sj.ki.5001760. [DOI] [PubMed] [Google Scholar]
- Lauer ME, Hascall VC, Wang A. Heparan sulfate analysis from diabetic rat glomeruli. J Biol Chem. 2007;282:843–852. doi: 10.1074/jbc.M608823200. [DOI] [PubMed] [Google Scholar]
- Mahadevan P, Larkins RG, Fraser JR, Fosang AJ, Dunlop ME. Increased hyaluronan production in the glomeruli from diabetic rats: a link between glucose-induced prostaglandin production and reduced sulphated proteoglycan. Diabetologia. 1995;38:298–305. doi: 10.1007/BF00400634. [DOI] [PubMed] [Google Scholar]