Abstract
The polycystic kidney (PCK) rat is an animal model of Caroli’s disease with congenital hepatic fibrosis, in which the mechanism of progressive hepatic fibrosis remains unknown. This study aimed to clarify the mechanism of hepatic fibrosis of the PCK rat from the viewpoint of the contribution of pathological cholangiocytes. In liver sections of the PCK rats, intrahepatic bile ducts were constituted by two different phenotypes: bile ducts lined by cuboidal-shaped and flat-shaped cholangiocytes. The flat-shaped cholangiocytes showed reduced immunohistochemical expression of the biliary epithelial marker cytokeratin 19 and positive immunoreactivity for vimentin and fibronectin. When cultured cholangiocytes of the PCK rat were treated with transforming growth factor (TGF)-β1, a potent inducer of epithelial-mesenchymal transition, induction of vimentin, fibronectin, and collagen expression occurred in the PCK cholangiocytes. Although the TGF-β1 treatment reduced cytokeratin 19 expression, the epithelial cell features characterized by the expression of E-cadherin and zonula occludens-1 was maintained, and α-smooth muscle actin expression was not induced in the cholangiocytes. Cholangiocytes of the PCK rat may acquire mesenchymal features in response to TGF-β1 and participate in progressive hepatic fibrosis by producing extracellular matrix molecules, which seems to be a different event from epithelial-mesenchymal transition.
The polycystic kidney (PCK) rat is an established animal model of Caroli’s disease with congenital hepatic fibrosis (CHF), as well as a slowly progressive model of autosomal recessive polycystic kidney disease (ARPKD).1 Caroli’s disease with CHF is a hepatic manifestation of ARPKD, and the hepatic lesions of ARPKD are characterized by multiple segmental and saccular dilation of the intrahepatic bile ducts and progressive and unresolving portal fibrosis.2,3,4 Persistence or lack of remodeling of the embryonic ductal plate is regarded as an essential precursor of the hepatic lesions of Caroli’s disease.5 Symptoms from the liver disease often result from complications of dilated ducts or hepatic fibrosis, and the spontaneous course of Caroli’s disease with CHF is dominated by biliary infection such as recurrent ascending cholangitis and sepsis.2,3,4
Mutations to orthologous genes, PKHD1/Pkhd1, have been identified in ARPKD patients and the PCK rat.6,7 Developing and mature intrahepatic bile ducts express the PKHD1 protein, fibrocystin, whereas bile ducts of ARPKD patients lack its expression.8 Mice with targeted mutation of Pkhd1 develop cystic biliary dysgenesis and portal fibrosis.9 In the PCK rat, cholangiocytes possess short and malformed cilia that do not express fibrocystin.10,11,12 Despite the identification of the genetic defect of the disease, the pathophysiology of the bile duct dilation and hepatic fibrosis are not fully understood. With regard to the mechanism of progressive dilation of intrahepatic bile ducts, our pervious studies have shown that activation of the signaling pathway mediated by epidermal growth factor receptor is involved in the abnormal cholangiocyte growth in the PCK rat.13,14
In most types of chronic liver diseases, activated hepatic stellate cells/myofibroblasts play major roles in hepatic fibrosis by producing extracellular matrix molecules.15 These fibrogenic processes usually occur after hepatocellular damage by various causative agents, whereas lack of necroinflammatory change in hepatic parenchyma is a histological feature of Caroli’s disease with CHF.2 In fact, our previous study demonstrated that α-smooth muscle actin (α-SMA)-expressing myofibroblasts were negligible in hepatic parenchyma in liver sections of patients with Caroli’s disease with CHF.16 Therefore, hepatic fibrosis in Caroli’s disease with CHF seems to be mediated by other cell types than hepatic stellate cells.
Recently, many studies have demonstrated that epithelial cells have an ability to acquire mesenchymal features, providing proof of principle for the process of epithelial-mesenchymal transition (EMT).17,18 EMT has been implicated in a variety of biological processes such as fibrogenesis as well as embryonic development and tumor progression. For example, renal tubular epithelial cells undergo EMT that is linked to the pathogenesis of renal interstitial fibrosis.19 In idiopathic pulmonary fibrosis, alveolar epithelial cells can serve as a source of myofibroblasts through EMT.20 Transforming growth factor (TGF)-β is known to be the most potent inducer of EMT, and it initiates morphological transition of the cells from an epithelial to a fibroblastic appearance, accompanied by a loss of epithelial cell markers such as E-cadherin and a gain of mesenchymal cell markers such as vimentin, fibronectin, and N-cadherin.18,19
According to our previous study,14 administration of a tyrosine kinase inhibitor inhibited intrahepatic bile duct dilation of the PCK rat, and this inhibition resulted in an improvement of hepatic fibrosis. In a rat model of biliary fibrosis, bile duct epithelial cells participate in the fibrotic process by producing connective tissue growth factor.21 In addition, a recent study has demonstrated that bile duct epithelial cells can undergo EMT, thereby contributing to hepatic fibrosis in a mouse model of bile duct ligation.22 These observations suggest that cholangiocytes contribute to hepatic fibrosis under several pathophysiological conditions, and the fibrocystic liver disease may be a candidate of such diseases. Using the PCK rat as an animal model of Caroli’s disease with CHF, this study was performed to clarify the mechanism of hepatic fibrosis of the PCK rat from the view point of the contribution of pathological cholangiocytes.
Materials and Methods
Animals and Tissues
PCK rats were maintained at the Laboratory Animal Institute of Kanazawa University Graduate School of Medicine. Normal (Crj:CD) rats were purchased from Charles River Japan (Sagamihara, Japan). Livers were removed from fetal (21 days of gestation), neonatal, and adult (1 day, 3 weeks, 2 months, 6 months, and 10 months old) rats. They were immersed in 10% formalin neutral buffer solution (pH 7.4) and embedded in paraffin. More than 10 serial sections, 4 μm thick, were cut from each paraffin block. Several of these sections were stained with hematoxylin and eosin (H&E) and Azan-Mallory, and the remainder was subjected to the immunohistochemical analysis. Parts of the tissue were immediately frozen in liquid nitrogen for use in the reverse transcriptase-polymerase chain reaction (RT-PCR) and an enzyme-linked immunosorbent assay (ELISA). All animal studies were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Takara-machi Campus of Kanazawa University, Kanazawa, Japan.
Cell Culture of PCK Cholangiocytes
Biliary epithelial cells were isolated, purified, and cultured from the intrahepatic large bile ducts of 8-week-old male PCK rats and normal rats as described previously.13 The 8th to 12th subcultured cholangiocytes were used for the study. Cells were set on type I collagen-coated cell culture dishes (Iwaki, Scitech Div., Chiba, Japan) covered with a standard culture medium composed of Dulbecco’s modified Eagle’s medium/F-12 (Life Technologies, Inc., Rockville, MD), 10% bovine growth serum (HyClone, Logan, UT), 1% ITS+ (Becton Dickinson, Bedford, MA), 5 μmol/L forskolin (Wako Pure Chemical, Osaka, Japan), 12.5 mg/ml of bovine pituitary extract (Kurabo Industries, Osaka, Japan), 1 μmol/L dexamethasone (Sigma, St. Louis, MO), 5 μmol/L triiodo-thyronine (Sigma), 5 mg/ml of glucose (Sigma), 25 mmol/L sodium bicarbonate (Sigma), 1% antibiotics-antimycotic (Life Technologies, Inc.), and 20 ng/ml of epidermal growth factor (Upstate Biotechnology, Lake Placid, NY) at 37°C in an atmosphere of 5% CO2. The standard culture medium was changed every 2 days until subconfluent.
Treatment of PCK Cholangiocytes with TGF-β1
At subconfluent state on type I collagen-coated cell culture dishes, cholangiocytes of PCK and normal rats were incubated with the standard medium or that containing TGF-β1 (2 ng/ml; R&D Systems, Inc., Minneapolis, MN) for 3 and 7 days. The culture medium was changed every day. After the treatment, the cultured cells and their supernatant were stored for the following experiments. To determine further the effects of direct cell contact with the basement membrane components, PCK cholangiocytes were cultured on type IV collagen- or laminin-coated cell culture dishes (BD Biosciences, Bedford, MA), and similarly treated with TGF-β1.
RT-PCR
Total RNA (1 μg) was extracted from the liver and cultured cholangiocytes using an RNA extraction kit (RNeasy mini; Qiagen, Tokyo, Japan) and was used to synthesize cDNA with reverse transcriptase (ReverTra Ace; Toyobo Co., Osaka, Japan). The sequences of the primers and conditions for PCR used are shown in Table 1. PCR amplification was performed in a reaction mixture containing 0.2 mmol/L dNTPs, 1 μmol/L each 5′- and 3′-primers, and 2.5 U of TaqDNA polymerase (Takara EX Taq; Takara Bio, Shiga, Japan). For each reaction, an initial denaturation cycle of 94°C for 3 minutes and a final cycle of 72°C for 10 minutes were incorporated. The PCR products were subjected to 2% agarose gel electrophoresis and stained with ethidium bromide.
Table 1.
Sequences of the Primers and PCR Conditions Used in This Study
| Gene | Sequences | Annealing temperature (°C) | PCR cycles |
|---|---|---|---|
| CK19 | 5′-GTACCAGAAGCAGGGACCCG-3′ | 60 | 22 |
| 5′-TTCCAGGGCAGCTTTCATGC-3′ | |||
| E-cadherin | 5′-GAGGGTTTTCACTGGTTGTT-3′ | 55 | 28 |
| 5′-GTAAAGACACTCTGGAGGTG-3′ | |||
| Vimentin | 5′-TTCAAGAACACCCGCACCAAC-3′ | 55 | 29 |
| 5′-GCCTTCCAGCAGCTTCCTGTA-3′ | |||
| Procollagen type I | 5′-CATAAAGGGTCATCGTGGCTTC-3′ | 65 | 35 |
| 5′-GTGATAGGTGATGTTCTGGGAG-3′ | |||
| Fibronectin | 5′-CCTACAACATCATAGTGGAG-3′ | 55 | 35 |
| 5′-CTTGTAGTTGACACCGTTGT-3′ | |||
| α-SMA | 5′-CATCCACGAAACCACCTATA-3′ | 55 | 40 |
| 5′-CTGTTTGCTGATCCACATCT-3′ | |||
| TGF-β1 | 5′-CAATTCCTGGCGTTACCTTG-3′ | 60 | 35 |
| 5′-GAAGCAGTAGTTGGTATCCA-3′ | |||
| TβR-II | 5′-GTGAAGAACGATTTGACCTG-3′ | 50 | 40 |
| 5′-GTTGTCTTTCATGCTCTCCA-3′ | |||
| β-Actin | 5′-ACCTTCAACACCCCAGCCATGTACG-3′ | 60 | 25 |
| 5′-CTGATCCACATCTGCTGGAAGGTGG-3′ |
CK, cytokeratin; SMA, smooth muscle actin; TGF, transforming growth factor; TβR-II, TGF-β type II receptor.
Immunofluorescence Confocal Microscopy
Cholangiocytes of PCK and normal rats grown on type I collagen-coated coverslips were treated with TGF-β1 (2 ng/ml) for 3 and 7 days. Then they were fixed with 4% paraformaldehyde for 15 minutes and permeabilized for 3 minutes with 0.1% Triton X-100. After blocking, the cells were incubated for 1 hour at room temperature with primary antibodies against pan-cytokeratin (CK) (1:600; DakoCytomation, Glostrup, Denmark), zonula occludens-1 (ZO-1) (1:200; Zymed, South San Francisco, CA), and vimentin (1:600, DakoCytomation). Alexa-488 and Alexa-568 (10 μg/ml; Molecular Probes, Eugene, OR) were used as a secondary antibody, and nuclei were stained with 4′,6-diamidino-2-phenylindole.
Western Blot Analysis
Proteins were extracted from cultured cholangiocytes using T-PER tissue protein extraction reagent (Pierce Chemical Co., Rockford, IL), and the total protein was measured spectrometrically. First, 50 μg of the protein was subjected to 10% sodium dodecyl sulfate-polyacrylamide electrophoresis and then electrophoretically transferred on to a nitrocellulose membrane. The membrane was incubated with primary antibodies against CK19 (1:100; Novocastra, Newcastle on Tyne, UK), E-cadherin (1:100, Zymed), and actin (1:3000; Abcam Inc., Cambridge, MA). The protein expression was detected using an EnVison+ system (DakoCytomation). 3,3′-Diaminobenzidine tetrahydrochloride was used as the chromogen. Semiquantitative analysis of the results was performed using the public domain NIH image software (National Institutes of Health, Bethesda, MD). The fold difference compared with actin expression was calculated.
ELISA
Concentrations of TGF-β1 were determined using an ELISA kit (R&D Systems) according to the manufacturer’s instructions. Briefly, serum and protein extracts from the liver of the PCK and normal rats were added to a 96-well plate coated with a monoclonal antibody for TGF-β1 and incubated for 2 hours at room temperature. After washing, an enzyme-linked polyclonal antibody for TGF-β1 was added and incubated for 2 hours. Color development was performed using a substrate solution, and its absorbance at 450 nm was measured. Total protein was measured spectrometrically, and TGF-β1/total protein concentration was calculated for each sample.
For the determination of concentration of fibronectin, an ELISA kit (Biomedical Technologies Inc, Stoughton, MA) was used. Briefly, cell culture supernatant was incubated with a specific primary antiserum against fibronectin in a 96-well plate for 1 hour at 37°C. For negative control wells, the standard culture medium was added. A second incubation was performed with an alkaline phosphatase-fibronectin conjugate. Separation of the antibody-bound and -free fractions was achieved by a second antibody that was precoated to the wells. After incubation with substrate, the amount of bound enzyme was determined by absorption at 405 nm.
Measurement of Collagen Content
The collagen content in cell culture supernatant was measured using the Sircol collagen assay kit (Biocolor Ltd., Belfast, UK) according to the manufacturer’s instructions. Briefly, Sirius red reagent (50 μl) was added to each culture supernatant (50 μl) and mixed for 30 minutes. The collagen-dye complex was precipitated by centrifugation at 15,000 × g for 5 minutes, washed with ethanol, and dissolved in 0.5 mol/L sodium hydroxide. Finally, the samples were introduced into a microplate reader, and the absorbance was determined at 540 nm. Because the standard culture medium contained detectable amounts of collagen, the data were expressed as an increase in collagen content by subtracting the measured value for the standard culture medium.
Immunohistochemistry
Immunostaining was performed for formalin-fixed, paraffin-embedded liver sections. After deparaffinization and blocking of the endogenous peroxidase, the sections were incubated overnight at 4°C with individual primary antibodies listed in Table 2. Then, the sections were incubated with secondary antibody conjugated to the peroxidase-labeled polymer, EnVison+ system (DakoCytomation). Color development was performed using 3,3′-diaminobenzidine tetrahydrochloride, and the sections were counterstained with hematoxylin. Control sections were evaluated by substitution of the primary antibodies with corresponding nonimmunized serum, which resulted in no signal detection.
Table 2.
Primary Antibodies Used for the Immunohistochemical Analysis
| Antibody specificity | Clone | Source | Dilution |
|---|---|---|---|
| Pan-CK | Polyclonal | DakoCytomation, Glostrup, Denmark | 1:600* |
| CK7 | OV-TL 12/30 | DakoCytomation | 1:50* |
| CK19 | b170 | Novocastra, Newcastle upon Tyne, UK | 1:100† |
| E-cadherin | 4A2C7 | Zymed, South San Francisco, CA | 1:200* |
| Vimentin | V9 | DakoCytomation | 1:600* |
| Desmin | D33 | DakoCytomation | 1:200 |
| α-SMA | 1A4 | DakoCytomation | 1:200 |
| N-cadherin | Polyclonal | Calbiochem, La Jolla, CA | 1:100* |
| Fibronectin | Polyclonal | DakoCytomation | 1:200‡ |
| TβR-I | Polyclonal | Santa Cruz Biotechnology, Inc., Santa Cruz, CA | 1:50† |
| TβR-II | Polyclonal | Santa Cruz Biotechnology, Inc. | 1:50† |
| Phospho-Smad2 | Polyclonal | Cell Signaling Technology, Inc., Danvers, MA | 1:100‡ |
Antigen retrieval was performed by microwaving in 10 mmol/L citrate buffer pH 6.0 (
), by incubating with 1 mg/ml trypsin for 15 minutes at 37°C, (
), and by incubating 20 mg/ml of proteinase K for 6 minutes at room temperature (
). CK, cytokeratin; SMA, smooth muscle actin; TβR-I, transforming growth factor-β type I receptor; TβR-II, TGF-β type II receptor.
Double Immunostaining and Histological Assessment
Double immunostaining of pan-CK and vimentin was conducted as follows. The deparaffinized liver sections were incubated overnight at 4°C with anti-pan-CK (1:200) and then incubated using the EnVision+ system (DakoCytomation). Color development was performed using the Vector Blue alkaline phosphatase substrate kit (Vector Laboratories, Burlingame, CA). The detection of vimentin was performed as above. Stained liver sections were visualized under a light microscope, and the digital images were acquired and reproduced on a computer. Areas of lumen of pan-CK single-positive bile ducts, and those of pan-CK- and vimentin-double-positive bile ducts were measured using image analysis software (WinROOF version 3.6; Mitani Corp., Tokyo, Japan). The areas of interest were expressed as a percentage of the total tissue.
The extent of fibrosis around bile ducts was determined using sections stained with Azan-Mallory. The digital images of the sections were reproduced on a computer, and a color threshold was applied at a level that separated periductal fibrosis from nonfibrotic tissue. Areas of periductal fibrosis and those of bile duct lumen were measured using WinROOF software (Mitani Corp.). Periductal fibrosis score was expressed as a ratio of area of periductal fibrosis/area of bile duct lumen.
Statistics
The mean ± SD was calculated for all parameters. Statistical differences were determined using the Mann-Whitney U-test or analysis of variance. A P value <0.05 was accepted as the level of statistical significance.
Results
Two Different Types of Intrahepatic Bile Ducts of the PCK Rat
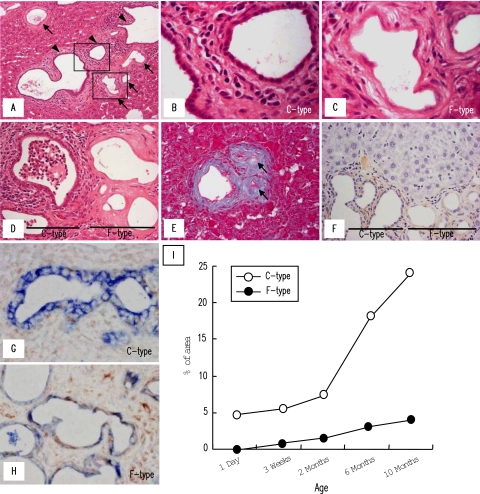
In liver sections of the PCK rats stained with H&E, two different types of intrahepatic bile ducts could be recognized, ie, bile ducts lined by cuboidal-shaped cholangiocytes [Figure 1, A (arrowheads) and B] and those lined by flat-shaped cholangiocytes [Figure 1, A (arrows) and C]. The bile ducts lined by cuboidal-shaped cholangiocytes (C-type) were a predominant phenotype in the liver, showing progressive cystic dilation with increasing age. The bile ducts lined by flat-shaped cholangiocytes (F-type) were not observed under the age of 1 day but seemed to distribute more frequently with advancing age. F-type had fusiform-shaped lumen with uneven nuclear spacing and lower nuclear density than those of C-type (Figure 1C). Some F-type bile ducts had enlarged nuclei. In the elderly rats, mildly dilated F-type bile ducts were observed, but F-type seemed not to show progressive cystic dilation (Figure 1A). At the ages of 6 and 10 months, cholangitis became a frequent histological finding in C-type with accumulation of polymorphonuclear leukocytes in their lumen. By contrast, F-type never associated with suppurative cholangitis even in the elderly rats, despite the close spatial distance between C- and F-types (Figure 1D).
Figure 1.
Two different types of intrahepatic bile ducts of the PCK rat. Intrahepatic bile ducts of the PCK rat consisted of bile ducts lined by cuboidal-shaped cholangiocytes (C-type) (A, arrowheads, and B) and those lined by flat-shaped cholangiocytes (F-type) (A, arrows, and C). B and C: High-power views of the squares outlined in A. D: Cholangitis with polymorphonuclear cell accumulation in the lumen was a frequent histological finding in C-type, whereas F-type never associated with suppurative inflammation. E: F-type occasionally showed fibrous scar-like appearance in the elderly rats (arrows). F: Immunostaining of α-SMA using PCK liver sections. G and H: Double immunostaining of pan-CK (colored by the Vector blue reaction) and vimentin (colored by the benzidine reaction) for the PCK livers. C-type bile ducts showed single-positive signal for pan-CK (G), whereas F-type bile ducts showed double-positive signals for pan-CK and vimentin with reduced expression of pan-CK (H). I: Frequency of distribution of C- and F-type bile ducts in the PCK liver. Morphometric analysis of the stained liver sections was performed as described in Materials and Methods. Representative photographs of the 6-month-old (A--C, G, H) and 10-month-old (D--F) PCK rats. A--D, H&E; E, Azan-Mallory. Original magnifications: ×200 (A); ×400 (D–F); ×1000 (B, C, G, H).
Both C- and F-types accompanied with progressive dense fibrosis around them, and F-type occasionally showed fibrous scar-like appearance in the elderly rats (Figure 1E, arrows). Despite dense fibrotic response in the portal tracts, α-SMA-expressing portal fibroblasts were scanty at any age of the rats around C- and F-type bile ducts (Figure 1F). Also, α-SMA-expressing myofibroblasts were negligible in hepatic parenchyma of the PCK rats (Figure 1F).
Frequency of Distribution of Two Different Type Bile Ducts in the PCK Liver during Aging
Double immunostaining of pan-CK (colored by the Vector Blue reaction) and vimentin (colored by the benzidine reaction) was performed for the PCK liver sections, and the frequency of distribution of C- and F-type bile ducts was determined. As demonstrated later, cholangiocytes constituting F-type bile ducts had mesenchymal features. In the analysis, pan-CK single-positive bile ducts (Figure 1G) were regarded as C-type, and bile ducts showing pan-CK and vimentin double-positive with reduced signal intensity of pan-CK (Figure 1H) were regarded as F-type. As shown in Figure 1I, the percentage of areas of C-type bile duct lumen to the whole liver tissue progressively increased with aging, and C-type was invariably predominant at any age of the rats. F-type first appeared at 3 weeks of age, and its frequency gradually increased up to 10 months of age.
Progressive Fibrosis around Two Different Type Bile Ducts in the PCK Liver during Aging
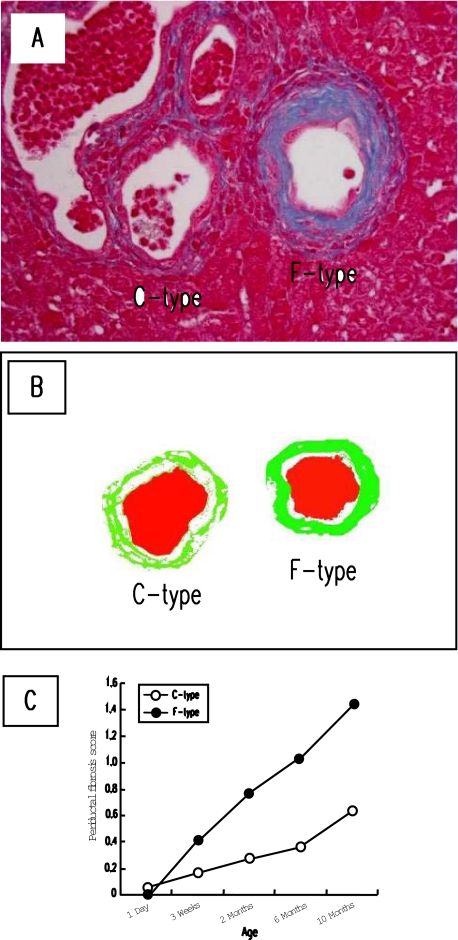
Fibrosis around the two different type bile ducts was assessed using liver sections stained with Azan-Mallory. C- and F-type bile ducts in Azan-Mallory-stained sections were determined using serial sections immunostained for pan-CK and vimentin. Fibrosis around F-type bile ducts tended to be more dense than that of C-type, and the image analysis could reproduce this tendency (Figure 2, A and B). As shown in Figure 2C, both C- and F-type bile ducts showed progressive fibrosis around them during aging, and the periductal fibrosis was more prominent around F-type bile ducts after 3 weeks of age.
Figure 2.
Progressive fibrosis around two different type bile ducts in the PCK liver during aging. A: Periductal fibrosis around F-type bile duct was more dense than that of C-type bile duct. To determine periductal fibrosis score, the image analysis was performed as described in Materials and Methods. B: Representative analyzed image of the figure shown in A (green, fibrotic area; red, bile duct lumen). C: Fibrosis occurred progressively around both C- and F-type bile ducts during aging, in which the extent was more prominent in F-type bile duct. A, Azan-Mallory. Original magnification, ×400.
Immunophenotype of Intrahepatic Bile Ducts of the PCK Rat
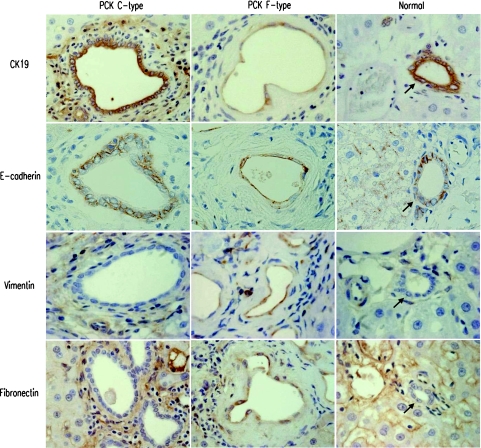
Immunohistochemical analysis showed that C-type bile ducts were diffusely and strongly positive for pan-CK and biliary epithelial markers (CK7, CK19), showing positive immunoreactivity for E-cadherin on their cell membrane (Figure 3, Table 3). Mesenchymal markers including vimentin and fibronectin were totally negative in C-type. The immunophenotype of C-type was identical with those of the intrahepatic bile ducts of adult normal rats. Similarly, bile duct epithelial cells of fetal livers showed the same immunophenotype in both PCK and normal rats (Table 3).
Figure 3.
Immunophenotype of intrahepatic bile ducts of the PCK rat. Immunostaining using liver sections for epithelial markers (CK19, E-cadherin) and mesenchymal markers (vimentin, fibronectin) was performed as described in Materials and Methods. Immunophenotype of C-type bile ducts of the PCK rats and interlobular bile ducts of the adult normal rats (arrows) was identical. In F-type bile ducts of the PCK rats, reduction of the expression of biliary epithelial marker CK19 was observed, whereas E-cadherin expression was unchanged. F-type showed immunoreactivity for mesenchymal markers, vimentin and fibronectin. Original magnifications, ×1000.
Table 3.
Summary of the Immunohistochemical Analysis for the Intrahepatic Bile Ducts of the PCK and Normal Rats
| PCK
|
Normal
|
|||||
|---|---|---|---|---|---|---|
| Fetal liver | Adult liver
|
Fetal liver | Adult liver | |||
| C-type | F- type | |||||
| Epithelial markers | Pan-CK | ++ | ++ | + | ++ | ++ |
| CK7 | ++ | ++ | + | ++ | ++ | |
| CK19 | ++ | ++ | + | ++ | ++ | |
| E-cadherin | − | + | + | − | + | |
| Mesenchymal markers | Vimentin | + | − | ++∼+ | + | − |
| Desmin | − | − | − | − | − | |
| α-SMA | − | − | − | − | − | |
| N-cadherin | − | − | + ∼− | − | − | |
| Fibronectin | − | − | ++ ∼+ | − | − | |
++, strongly positive; +, moderate to weakly positive; −, negative. CK, cytokeratin; SMA, smooth muscle actin.
In F-type of the PCK rat, reduction of pan-CK, CK7, and CK19 expression was observed. E-cadherin expression appeared to be maintained in F-type, although cell membranous localization and borders between adjacent cholangiocytes became unclear (Figure 3). F-type showed positive immunoreactivity for mesenchymal markers such as vimentin and fibronectin (Figure 3, Table 3). Occasionally, the expression of N-cadherin was observed in F-type. Bile ducts of both C- and F-types lacked α-SMA expression (Figure 1F).
Expression of TGF-β1 and Its Receptors in the PCK Rat
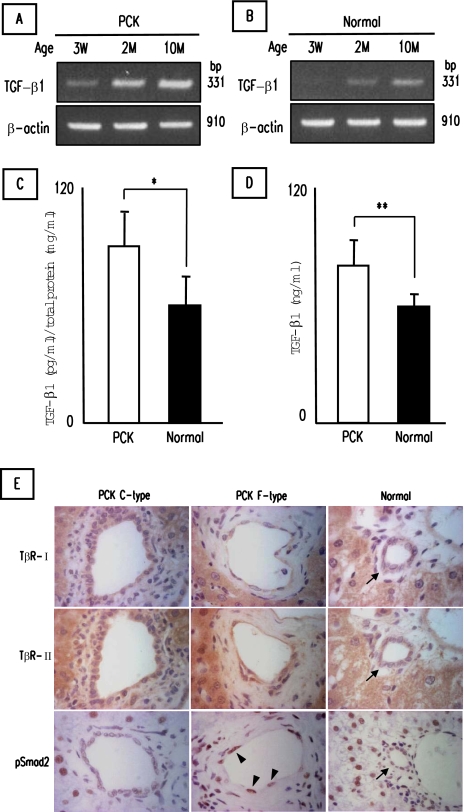
To determine the possibility of the occurrence of EMT in PCK cholangiocytes, in vitro studies using cultured cholangiocytes were performed. Before the analysis, in vivo expression of TGF-β1, a potent inducer of EMT, and receptors for TGF-β1 were examined. RT-PCR analysis showed that whole liver of the PCK rats expressed TGF-β1 mRNA, and the expression level increased with aging (Figure 4A), which was in accordance with the increase in the appearance of cholangiocytes with mesenchymal features (F-type) along with aging (Figure 1I). Livers of normal rats also expressed TGF-β1 mRNA, and the expression level tended to be lower when compared to those of the PCK rat (Figure 4B). At the protein level, the PCK rat expressed a significantly high level of TGF-β1 in the whole liver (Figure 4C) and the serum (Figure 4D) at 10 months of age.
Figure 4.
Expression of TGF-β1 and its receptors in the PCK rat. A and B: RT-PCR analysis for TGF-β1 mRNA expression in the whole liver of the PCK (A) and normal (B) rats at different age. Total RNA extracted from the whole liver was used for the analysis. C and D: TGF-β1 concentration in protein extracts from the livers (C) and the serum (D) of the PCK and normal rats at 10 months of age. TGF-β1 concentration in the samples was determined using ELISA. E: Immunostaining of TGF-β type I receptor (TβR-I), TGF-β type II receptor (TβR-II), and phospho-Smad2 (pSmad2). TβR-I and TβR-II were expressed in bile ducts of C- and F-types of the PCK rats and those of normal rats (arrows). Nuclei of cholangiocytes of F-type bile duct showed strong immunoreactivity for pSmad2 (arrowheads), whereas the staining was totally weak or invisible in C-type and normal bile ducts. The data represent three independent experiments (A, B) and the mean ± SD of four per group (C). *P < 0.05, **P < 0.01.
Bile ducts of C- and F-types of the PCK rats and those of normal rats invariably expressed TGF-β type I receptor (TβR-I) and TGF-β type II receptor (TβR-II) to the same extent, with the exception of reduced signals of TβR-I in F-type bile ducts (Figure 4E). By contrast, dilated bile ducts at the hilar region of the PCK rats showed increased positive signals of TβR-I (data not shown). Hepatocytes also showed diffuse cytoplasmic staining of TβR-I and TβR-II in both rats. Nuclei of cholangiocytes of F-type bile duct showed strong immunoreactivity for phospho-Smad2 (pSmad2), whereas the staining was totally weak or invisible in C-type and normal bile ducts (Figure 4E). Hepatocytes of both PCK and normal rats were diffusely labeled with the anti-pSmad2 antibody used in this study.
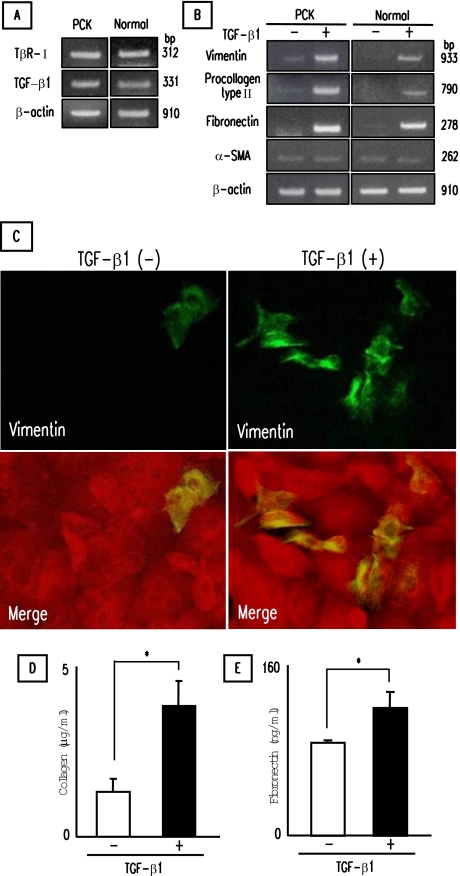
Induction of Mesenchymal Markers in PCK Cholangiocytes by TGF-β1
Cultured PCK and normal cholangiocytes were stimulated with TGF-β1 for 3 days on type I collagen-coated cell culture dishes, and the expression of mesenchymal markers was examined. Experiments using RT-PCR showed that PCK and normal cholangiocytes expressed mRNA for TβR-II (Figure 5A). PCK and normal cholangiocytes also contained detectable amounts of TGF-β1 mRNA. After stimulation, an increase in the expression of mRNA for vimentin, procollagen type I, and fibronectin was observed in PCK and normal cholangiocytes by RT-PCR, but α-SMA mRNA expression was unchanged (Figure 5B). On immunofluorescence confocal microscopy, the majority of PCK and normal cholangiocytes were negative for vimentin, and TGF-β1 increased the number of vimentin-positive cholangiocytes (Figure 5C). In accordance with the induction of mRNA of procollagen type I and fibronectin, cell culture supernatant of the PCK cholangiocytes contained significantly increased amounts of collagen (Figure 5D) and fibronectin (Figure 5E) after the treatment. Prolonged incubation (7 days) with TGF-β1 resulted in almost identical results (data not shown).
Figure 5.
Induction of mesenchymal markers in PCK cholangiocytes by TGF-β1. Cultured cholangiocytes were incubated with TGF-β1 for 3 days on type I collagen-coated cell culture dishes. A: Detection of TGF-β type II receptor (TβR-II) and TGF-β1 mRNA in PCK and normal cholangiocytes using RT-PCR. B: Effects of TGF-β1 on the expression of mesenchymal markers in cultured cholangiocytes determined by RT-PCR. C: Induction of vimentin in PCK cholangiocytes by TGF-β1. Vimentin was visualized by Alexa-488 (green), and pan-CK was visualized by Alexa-568 (red) under immunofluorescence confocal microscopy. Bottom panels were merged images of vimentin and pan-CK. D and E: Concentrations of collagen (D) and fibronectin (E) in cell culture supernatant of PCK cholangiocytes. The concentrations of collagen and fibronectin were determined as described in Materials and Methods. The data represent three independent experiments (A, B) and the mean ± SD of four per group (D, E). *P < 0.01. Original magnifications, ×1000.
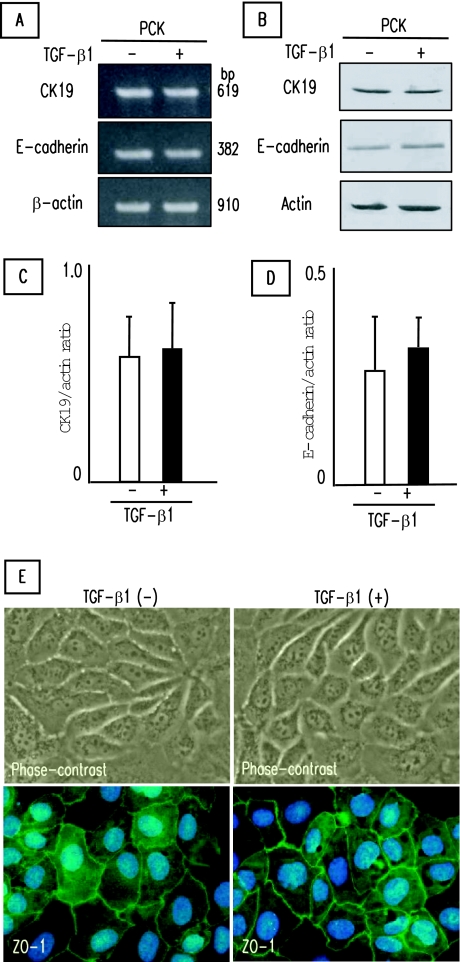
Effects of TGF-β1 on Epithelial Cell Phenotype of PCK Cholangiocytes
PCK cholangiocytes treated with TGF-β1 for 3 days on type I collagen-coated cell culture dishes were used for the analysis of epithelial cell phenotype. As shown in Figure 6A, CK19 and E-cadherin mRNA expression was not affected by the TGF-β1 treatment in the PCK cholangiocytes by RT-PCR analysis. Western blot analysis also showed no effects of TGF-β1 on CK19 and E-cadherin expression in the PCK cholangiocytes at the protein level (Figure 6B). Semiquantitative analysis of the results of Western blotting confirmed no significant inhibitory effects of TGF-β1 on the expression of epithelial markers (Figure 6, C and D). Under the phase-contrast microscope, epithelial cell morphology was maintained in PCK cholangiocytes after TGF-β1 treatment, and no morphological transition of the cells from an epithelial to a fibroblastic appearance was observed (Figure 6E). In addition, tight junctions between adjacent cells were preserved after the treatment as shown by the expression of ZO-1 (Figure 6E). Similar results were obtained for the normal cholangiocytes. Reduction of CK19 and E-cadherin expression was not observed after TGF-β1 treatment for a longer period (7 days) in the PCK and normal cholangiocytes (data not shown).
Figure 6.
Effects of TGF-β1 on epithelial cell phenotype of PCK cholangiocytes. Cultured PCK cholangiocytes were incubated with TGF-β1 for 3 days on type I collagen-coated cell culture dishes. A and B: No inhibitory effects of TGF-β1 on the expression of CK19 and E-cadherin determined by RT-PCR (A) and Western blotting (B). C and D: Semiquantitative analysis of the results of Western blotting was performed for CK19 (C) and E-cadherin (D), confirming no significant inhibitory effects of TGF-β1. E: No effects of TGF-β1 on epithelial cell morphology of PCK cholangiocytes. E: Morphological transition was not observed after TGF-β1 treatment under the phase-contrast microscope (top). E: Tight junctions were present even between adjacent cells after the TGF-β1 treatment as shown by expression of ZO-1 (bottom). ZO-1 was visualized by Alexa-488 (green) under immunofluorescence confocal microscopy. Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). The data represent three independent experiments (A) and the mean ± SD of four per group (C, D). Original magnifications, ×1000.
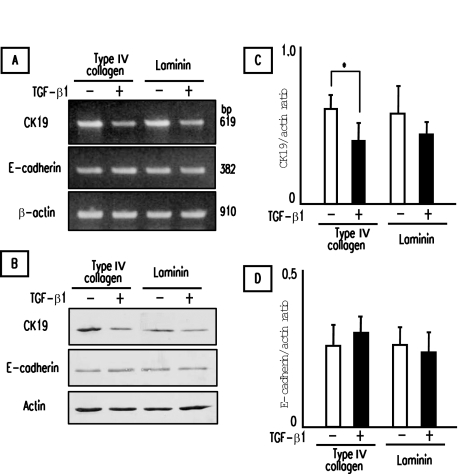
Reduction of Biliary Epithelial Phenotype of PCK Cholangiocytes by TGF-β1—Effects of Cell Contact with Basement Membrane Components
Immunohistochemical analysis showed reduced expression of biliary epithelial marker CK19 in F-type bile ducts of the PCK rat (Figure 3, Table 3). However, in vitro analysis failed to demonstrate reduction of CK19 expression after TGF-β1 treatment in the PCK cholangiocytes cultured on type I collagen-coated cell culture dishes. Because cell contact with basement membrane components such as type IV collagen and laminin is an important developmental and biological microenvironment for bile duct epithelial cells, PCK cholangiocytes were cultured on type IV collagen- and laminin-coated cell culture dishes, and the effects of TGF-β1 on biliary epithelial phenotype were examined.
After a 3-day stimulation with TGF-β1, the expression of CK19 mRNA appeared to be reduced in the PCK cholangiocytes cultured on type IV collagen and laminin, whereas E-cadherin mRNA expression was unchanged (Figure 7A). Western blot analysis showed that TGF-β1 significantly reduced CK19 expression in the PCK cholangiocytes cultured on type IV collagen (Figure 7, B and C). E-cadherin expression of the PCK cholangiocytes cultured on type IV collagen and laminin was not significantly affected by TGF-β1 at the protein level (Figure 7D), consistent with the immunohistochemical results of no reduction of E-cadherin expression in F-type bile ducts (Figure 3).
Figure 7.
Reduction of biliary epithelial phenotype of PCK cholangiocytes by TGF-β1. Effects of cell contact with basement membrane components were determined using PCK cholangiocytes cultured on type IV collagen- and laminin-coated cell culture dishes. The cells were incubated with TGF-β1 for 3 days. A and B: The expression of CK19 and E-cadherin in PCK cholangiocytes were determined by RT-PCR (A) and Western blotting (B). C and D: Semiquantitative analysis of the results of Western blotting for CK19 (C) and E-cadherin (D). TGF-β1 significantly inhibited the CK19 expression in the presence of type IV collagen (C), whereas E-cadherin expression was unaffected (D). The data represent the mean ± SD of four per group (C, D). *P < 0.05.
Discussion
This study demonstrated the involvement of pathological cholangiocytes in progressive portal fibrosis of the PCK rat. Intrahepatic bile ducts of the PCK rat constituted by the two distinct phenotypes, C- and F-types, in which C-type bile ducts were the predominant phenotype in the liver. The F-type bile ducts appeared more frequently with advancing age, suggesting acquired nature of the development of F-type bile ducts in the PCK liver. Immunohistochemical analysis for the PCK liver sections revealed that C-type bile ducts had an identical immunophenotype with that of intrahepatic bile ducts of the adult normal rats. By contrast, F-type showed reduced expression of biliary epithelial marker CK19 and positivity for mesenchymal markers vimentin and fibronectin. The results of the immunohistochemical analysis indicate that cholangiocytes with mesenchymal features (F-type) contribute to progressive hepatic fibrosis of the PCK rat. The small number of cells of α-SMA-expressing myofibroblasts or fibroblasts in the PCK liver also suggests involvement of pathological cholangiocytes, rather than hepatic stellate cells and residential portal fibroblasts, in the process of hepatic fibrosis of the PCK rat.
In several types of chronic fibrotic disorders, epithelial cells acquire mesenchymal features, thereby contributing to fibrogenic process, a phenomena known as EMT.17,18 EMT has been also implicated in embryonic development and tumor progression. EMT contributes to the degeneration of epithelial structures and to the generation of fibroblasts associated with accumulation of extracellular matrix in chronic fibrotic disorders, particularly in renal interstitial fibrosis. The key steps of EMT include loss of epithelial cell adhesion and de novo α-SMA expression.19 The expression of E-cadherin, a central component of cell-cell adhesion junctions, is usually lost at sites of EMT during development and cancer.17,23 The results of in vivo analysis of this study showed reduction of CK19 expression and a gain of the mesenchymal features of the F-type bile ducts of the PCK rat. However, E-cadherin expression was not reduced, and α-SMA expression was not observed in F-type bile ducts in vivo. These in vivo results of this study seemed not to fulfill the features of EMT. However, a recent study has demonstrated that TGF-β1 induces α-SMA and fibronectin expression, and suppresses CK19 in cultured biliary epithelial cells, providing evidence of the occurrence of EMT in noncancerous cholangiocytes.22 We therefore addressed whether PCK cholangiocytes were able to undergo EMT in vitro.
First, we examined phenotypic changes of PCK and normal cholangiocytes cultured on type I collagen-coated cell culture dishes after TGF-β1 treatment. As expected, TGF-β1 induced the expression of vimentin, collagen, and fibronectin in PCK cholangiocytes. However, the expression of CK19 and E-cadherin was unaffected by the treatment. Consistent with these results, morphological alterations of the cholangiocytes from an epithelial to a fibroblastic appearance were not observed under the phase-contrast microscope, and tight junctions between adjacent cells were well preserved after the treatment as assessed by the expression of ZO-1. In vivo, F-type bile ducts showed reduced expression of CK19, but in vitro analysis failed to demonstrate reduction of the CK19 expression in the cholangiocytes cultured on type I collagen-coated cell culture dishes by TGF-β1. Because cell contact to the basement membrane component is an important microenvironment that provides structural and functional support to various cell types including cholangiocytes,24,25,26 we next examined the effects of the cell contact to a major component of basement membranes, type IV collagen, and laminin, on the epithelial cell phonotype of the PCK cholangiocytes after TGF-β1 treatment.
As shown in Figure 7, TGF-β1 reduced CK19 expression in the PCK cholangiocytes in the presence of type IV collagen. However, E-cadherin expression was unchanged in the presence of the basement membrane components after TGF-β1 treatment. The reduction of CK19 expression and the maintenance of E-cadherin expression in the PCK cholangiocytes were consistent with the immunophenotype of F-type bile ducts in vivo. Type IV collagen is the most abundant protein in basement membranes and acts as a scaffold to provide structural and functional integrity.27 In renal tubular epithelial cells, it has been reported that type IV collagen contributes to the maintenance of the epithelial cell phenotype, whereas type I collagen promotes EMT in response to TGF-β1.26 Our data showed that TGF-β1 reduced biliary epithelial cell phenotype in the PCK cholangiocytes in the presence of type IV collagen, but the epithelial cell phenotype characterized by E-cadherin expression was not significantly affected by TGF-β1.
The results of this study indicate that PCK cholangiocytes do not undergo EMT by the TGF-β1 treatment. Although there is growing interest in TGF-β1-mediated EMT, the phenotype that undergoes EMT is limited to only a few cell lines in vitro. According to previous studies, only 2 of 26 cell lines underwent TGF-β1-mediated EMT, which was assessed by cell morphology and localization of ZO-1, E-cadherin, and F-actin.28 Although the role of myofibroblasts in renal fibrosis is widely accepted, their origin and fate are still debated.19,29 Recently, it has been shown that kidney tubular epithelial cells acquire mesenchymal features in response to TGF-β1, but they accompanied no de novo expression of α-SMA.30 Thus, TGF-β1 does not necessarily induce myofibroblast transdifferentiation in a certain cell types including the PCK cholangiocytes, and in these cell lines TGF-β1 may act as an inducer initiating a cascade of dedifferentiating events.
Immunohistochemical analysis demonstrated the presence of TβR-I and TβR-II in both C- and F-type bile ducts as well as normal bile ducts in vivo. Our previous finding showing that cultured PCK cholangiocytes expressed an increased amount of TβR-I mRNA might relate to the immunohistochemical result of the increased expression of TβR-I in the dilated bile ducts at the hilar region of the PCK rat in the present study because cultured PCK cholangiocytes were obtained from the intrahepatic large bile ducts.13 Although we have proposed the acquired nature of the development of F-type bile ducts in the PCK liver during aging, the immunohistochemical analysis of TβR-I and TβR-II expression failed to explain why a selected portion of intrahepatic bile ducts of the PCK rat underwent the dedifferentiating events. Interestingly, many cholangiocytes of F-type bile duct showed immunohistochemical expression of pSmad2, suggesting the transmission of TGF-β signals from the cell surface into the nucleus. It is still unclear why TGF-β signaling occurred in some, but not all, cholangiocytes in the PCK liver. Other molecules involved in the TGF-β signaling such as Smad4 may associate with this process, and, therefore, further study is necessary.
The expression of vimentin and the reduction of CK19 expression in F-type bile ducts of the PCK rat are similar to the immunophenotype of proliferating bile ductules in nonneoplastic hepatobiliary diseases.31,32 Bile ductules are supposed to arise via transdifferentiation or metaplasia of hepatocytes, and they are occasionally continuous or admixed with the periportal hepatocytes.33 F-type bile ducts of the PCK rat are surrounded by dense fibrous stroma and never show direct connections to the periportal hepatocytes, suggesting that transdifferentiation or metaplasia of hepatocytes does not account for the development of F-type bile ducts.
The fetal liver usually contains cells in the status of EMT associated with embryogenesis, and the EMT cells disappear by the end of gestation and in the adult.34 Recent studies have shown that human and rodent cholangiocytes in the polycystic liver diseases have features that are similar to fetal ductal plate cells.35 In this study, immunophenotype of the bile ducts was different between the fetal liver and F-type of the adult PCK liver. These results indicate that TGF-β1 may initiate a cascade of dedifferentiating events in the PCK cholangiocytes but may not act as an embryonal inducer.
Based on the morphological appearance of the F-type bile ducts in PCK liver sections, another possible mechanism of development of F-type bile ducts is cellular senescence of the cholangiocytes. Cellular senescence is defined as a condition in which a cell no longer has the ability to proliferate, and senescent cells display histological features such as cytoplasmic eosinophilia, cellular and nuclear enlargement, and uneven nuclear spacing.36 Recent studies have focused on the cellular senescence of biliary epithelial cells in several biliary disorders.37,38 In this study, F-type bile ducts did not show progressive cystic dilation and had enlarged nuclei and uneven nuclear spacing. However, F-type lacked cytoplasmic eosinophilia and cellular enlargement, and the expression of senescence-associated β-galactosidase activity, which is frequently associated with senescent cells, was not observed in the F-type bile ducts (unpublished data). Thus, cellular senescence is unlikely for the development of F-type bile ducts.
In the elderly rats, suppurative cholangitis was a frequent histological finding in C-type, whereas F-type never associated with cholangitis with accumulation of polymorphonuclear leukocytes in their lumen. In addition, F-type occasionally showed fibrous scar-like appearance. Recent studies have shown that the majority of dilated intrahepatic bile ducts of the PCK rat are initially connected to the biliary tree, but throughout time separates from them resulting in true cyst formation.11 Our results indicate that F-type is the bile ducts that are disconnected to the biliary tree and may account for the observation of the previous report showing the presence of true biliary cysts in the aged PCK rats.11
In summary, this study demonstrated that cholangiocytes of the PCK rat were constituted by two distinct phenotypes and elucidated the contribution of pathological cholangiocytes to progressive hepatic fibrosis. In the PCK liver, cholangiocytes probably acquire mesenchymal features during aging and participate in progressive hepatic fibrosis by producing extracellular matrix molecules. In the acquisition of mesenchymal features of the cholangiocytes, TGF-β1 may act as an inducer initiating a cascade of dedifferentiating events, which are not directed toward myofibroblast transdifferentiation, and thus a different event from EMT. Our results show that pathological cholangiocytes of the PCK rat are closely associated with hepatic fibrosis. This implies that inhibition of abnormal cystic growth of the cholangiocytes leads to improvement of liver fibrosis, which may represent an important aspect in dealing with therapeutic interventions of ARPKD.
Footnotes
Address reprint requests to Yasuni Nakanuma, M.D., Ph.D., Department of Human Pathology, Kanazawa University, Graduate School of Medicine, Kanazawa 920-8640, Japan. E-mail: pbcpsc@kenroku.kanazawa-u.ac.jp.
References
- Sanzen T, Harada K, Yasoshima M, Kawamura Y, Ishibashi M, Nakanuma Y. Polycystic kidney rat is a novel animal model of Caroli’s disease associated with congenital hepatic fibrosis. Am J Pathol. 2001;158:1605–1612. doi: 10.1016/S0002-9440(10)64116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanuma Y, Terada T, Ohta G, Kurachi M, Matsubara F. Caroli’s disease in congenital hepatic fibrosis and infantile polycystic disease. Liver. 1982;2:346–354. doi: 10.1111/j.1600-0676.1982.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol. 2006;2:40–55. doi: 10.1038/ncpneph0070. [DOI] [PubMed] [Google Scholar]
- Sweeney WE, Jr, Avner ED. Molecular and cellular pathophysiology of autosomal recessive polycystic kidney disease (ARPKD). Cell Tissue Res. 2006;326:671–685. doi: 10.1007/s00441-006-0226-0. [DOI] [PubMed] [Google Scholar]
- Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- Moser M, Matthiesen S, Kirfel J, Schorle H, Bergmann C, Senderek J, Rudnik-Schoneborn S, Zerres K, Buettner R. A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD). Hepatology. 2005;41:1113–1121. doi: 10.1002/hep.20655. [DOI] [PubMed] [Google Scholar]
- Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, Punyashthiti R, Ritman EL, Torres VE, Harris PC, LaRusso NF. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology. 2003;125:1303–1310. doi: 10.1016/j.gastro.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Masyuk TV, Huang BQ, Masyuk AI, Ritman EL, Torres VE, Wang X, Harris PC, Larusso NF. Biliary dysgenesis in the PCK rat, an orthologous model of autosomal recessive polycystic kidney disease. Am J Pathol. 2004;165:1719–1730. doi: 10.1016/S0002-9440(10)63427-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muff MA, Masyuk TV, Stroope AJ, Huang BQ, Splinter PL, Lee SO, Larusso NF. Development and characterization of a cholangiocyte cell line from the PCK rat, an animal model of autosomal recessive polycystic kidney disease. Lab Invest. 2006;86:940–950. doi: 10.1038/labinvest.3700448. [DOI] [PubMed] [Google Scholar]
- Sato Y, Harada K, Kizawa K, Sanzen T, Furubo S, Yasoshima M, Ozaki S, Ishibashi M, Nakanuma Y. Activation of the MEK5/ERK5 cascade is responsible for biliary dysgenesis in a rat model of Caroli’s disease. Am J Pathol. 2005;166:49–60. doi: 10.1016/S0002-9440(10)62231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Harada K, Furubo S, Kizawa K, Sanzen T, Yasoshima M, Ozaki S, Isse K, Sasaki M, Nakanuma Y. Inhibition of intrahepatic bile duct dilation of the polycystic kidney rat with a novel tyrosine kinase inhibitor gefitinib. Am J Pathol. 2006;169:1238–1250. doi: 10.2353/ajpath.2006.051136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki S, Sato Y, Yasoshima M, Harada K, Nakanuma Y. Diffuse expression of heparan sulfate proteoglycan and connective tissue growth factor in fibrous septa with many mast cells relate to unresolving hepatic fibrosis of congenital hepatic fibrosis. Liver Int. 2005;25:817–828. doi: 10.1111/j.1478-3231.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158:1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168:1500–1512. doi: 10.2353/ajpath.2006.050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Terada T, Nakanuma Y. Expression of tenascin, type IV collagen and laminin during human intrahepatic bile duct development and in intrahepatic cholangiocarcinoma. Histopathology. 1994;25:143–150. doi: 10.1111/j.1365-2559.1994.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Satoh T, Sasatomi E, Miyazaki K, Tokunaga O. Critical role of type IV collagens in the growth of bile duct carcinoma. In vivo and in vitro studies. Pathol Res Pract. 2001;197:585–596. doi: 10.1078/0344-0338-00132. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001;159:1313–1321. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981;120:203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Brown KA, Aakre ME, Gorska AE, Price JO, Eltom SE, Pietenpol JA, Moses HL. Induction by transforming growth factor-beta1 of epithelial to mesenchymal transition is a rare event in vitro. Breast Cancer Res. 2004;6:R215–R231. doi: 10.1186/bcr778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JL, Szcykalski LM, Springer F, Barnes JL. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am J Pathol. 2005;167:1193–1205. doi: 10.1016/S0002-9440(10)61208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forino M, Torregrossa R, Ceol M, Murer L, Vella MD, Prete DD, D’Angelo A, Anglani F. TGFbeta1 induces epithelial-mesenchymal transition, but not myofibroblast transdifferentiation of human kidney tubular epithelial cells in primary culture. Int J Exp Pathol. 2006;87:197–208. doi: 10.1111/j.1365-2613.2006.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanuma Y, Kono N. Expression of vimentin in proliferating and damaged bile ductules and interlobular bile ducts in nonneoplastic hepatobiliary diseases. Mod Pathol. 1992;5:550–554. [PubMed] [Google Scholar]
- Harada K, Kono N, Tsuneyama K, Nakanuma Y. Cell-kinetic study of proliferating bile ductules in various hepatobiliary diseases. Liver. 1998;18:277–284. doi: 10.1111/j.1600-0676.1998.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Doi Y, Watanabe H, Tokairin T, Omori Y, Su M, Yoshioka T, Enomoto K. Transdifferentiation of mature rat hepatocytes into bile duct-like cells in vitro. Am J Pathol. 2005;166:1077–1088. doi: 10.1016/S0002-9440(10)62328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagraoui J, Lepage-Noll A, Anjo A, Uzan G, Charbord P. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood. 2003;101:2973–2982. doi: 10.1182/blood-2002-05-1341. [DOI] [PubMed] [Google Scholar]
- Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, Sonzogni A, Joplin RE, Okolicsanyi L, Strazzabosco M. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- Lunz JG, III, Contrucci S, Ruppert K, Murase N, Fung JJ, Starzl TE, Demetris AJ. Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: increased expression of p21(WAF1/Cip1) as a disease marker and the influence of immunosuppressive drugs. Am J Pathol. 2001;158:1379–1390. doi: 10.1016/S0002-9440(10)64089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Ikeda H, Sato Y, Nakanuma Y. Decreased expression of Bmi1 is closely associated with cellular senescence in small bile ducts in primary biliary cirrhosis. Am J Pathol. 2006;169:831–845. doi: 10.2353/ajpath.2006.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Ikeda H, Haga H, Manabe T, Nakanuma Y. Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J Pathol. 2005;205:451–459. doi: 10.1002/path.1729. [DOI] [PubMed] [Google Scholar]