Abstract
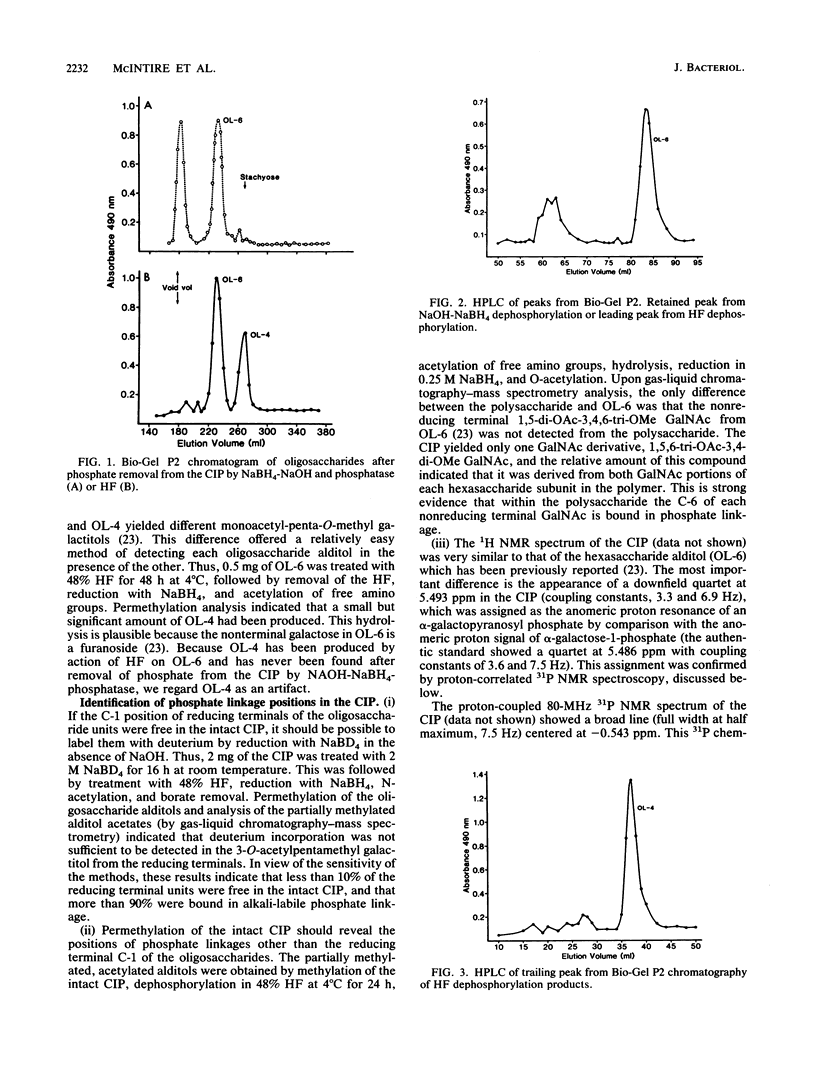
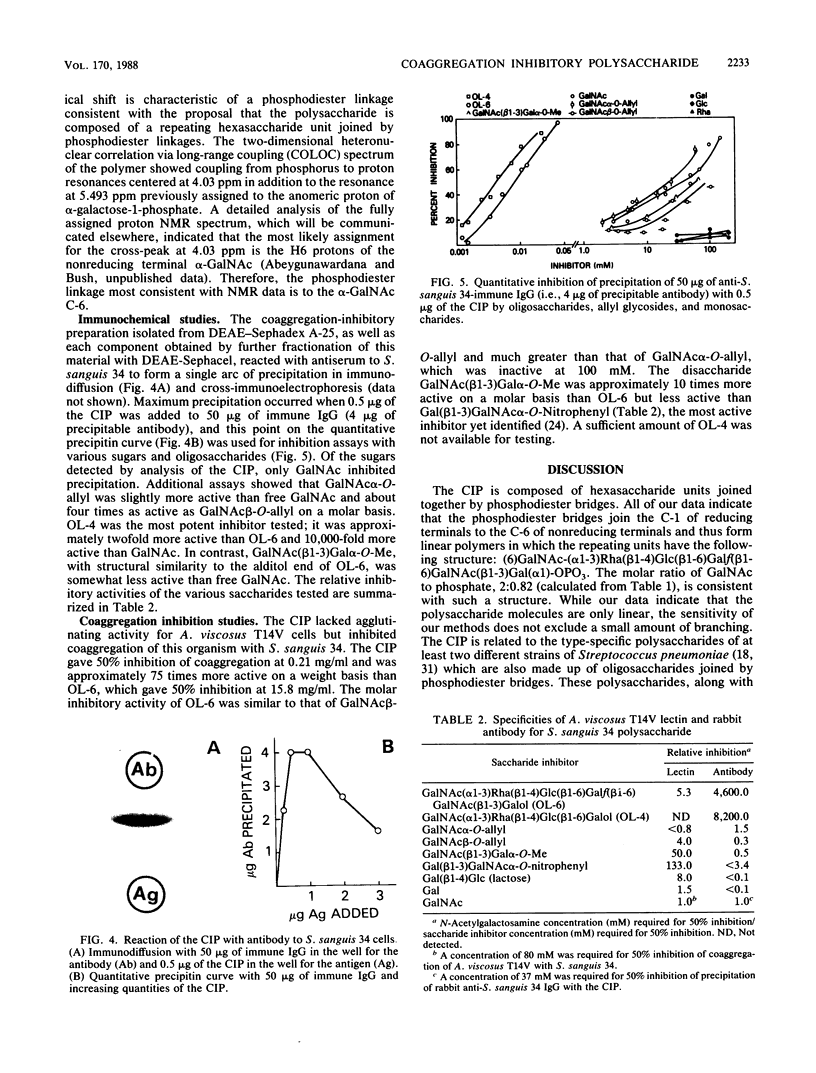
Coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34 depends on interaction of a lectin on A. viscosus T14V with a cell surface carbohydrate on S. sanguis 34. This carbohydrate was isolated, and its chemical makeup was established. The carbohydrate remained attached to S. sanguis 34 cells through extraction with Triton X-100 and treatment with pronase. It was cleaved from the cell residue by autoclaving and purified by differential centrifugation and column chromatography on DEAE-Sephacel and Sephadex G-75. The polysaccharide contained phosphate which was neither inorganic nor monoester. Treatment with NaOH-NaBH4, followed by Escherichia coli alkaline phosphatase, or with 48% HF at 4 degrees C, followed by NaBH4, yielded inorganic phosphate and oligosaccharide alditols. Therefore, the polysaccharide is composed of oligosaccharide units joined together by phosphodiester bridges. The structure and stereochemistry of the main oligosaccharide alditol was established previously (F. C. McIntire, C. A. Bush, S.-S. Wu, S.-C. Li, Y.-T. Li, M. McNeil, S. Tjoa, and P. V. Fennessey, Carbohydr. Res. 166:133-143). Permethylation analysis, 1H and 31P nuclear magnetic resonance studies on the whole polysaccharide revealed the position of the phosphodiester linkages. The polysaccharide is mainly a polymer of (6) GalNAc(alpha 1-3)Rha(beta 1-4)Glc(beta 1-6)Galf(beta 1-6)GalNAc(beta 1- 3)Gal(alpha 1)-OPO3. It reacted as a single antigen with antiserum to S. sanguis 34 cells and was a potent inhibitor of coaggregation between A. viscosus T14V and S. sanguis 34. Quantitative inhibition of precipitation assays with oligosaccharides, O-allyl N-acetylgalactosaminides, and simple sugars indicated that specific antibodies were directed to the GalNAc end of the hexasaccharide unit. In contrast, coaggregation was inhibited much more effectively by saccharides containing betaGalNAc. Thus, the specificity of the A. viscosus T14V lectin is strikingly different from that of antibodies directed against the S. sanguis 34 polysaccharide.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Joralmon R. A., Cisar J. O., Sandberg A. L. Binding of Actinomyces naeslundii to glycosphingolipids. Infect Immun. 1987 Feb;55(2):487–489. doi: 10.1128/iai.55.2.487-489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. K., Knox K. W., Wicken A. J. Extractability of cell wall polysaccharide from lactobacilli and streptococci by autoclaving and by dilue acid. Infect Immun. 1978 Dec;22(3):842–851. doi: 10.1128/iai.22.3.842-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Curl S. H., Kolenbrander P. E., Vatter A. E. Specific absence of type 2 fimbriae on a coaggregation-defective mutant of Actinomyces viscosus T14V. Infect Immun. 1983 May;40(2):759–765. doi: 10.1128/iai.40.2.759-765.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J., Kabat E. A., Dorner M. M., Liao J. Binding properties of immunoglobulin combining sites specific for terminal or nonterminal antigenic determinants in dextran. J Exp Med. 1975 Aug 1;142(2):435–459. doi: 10.1084/jem.142.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER L., BURGER M. M. THE SYNTHESIS OF TEICHOIC ACIDS. 3. GLUCOSYLATION OF POLYGLYCEROPHOSPHATE. J Biol Chem. 1964 Oct;239:3187–3191. [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Heeb M. J., Marini A. M., Gabriel O. Factors affecting binding of galacto ligands to Actinomyces viscosus lectin. Infect Immun. 1985 Jan;47(1):61–67. doi: 10.1128/iai.47.1.61-67.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen E., Jennings H. J. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type 19A (57). Carbohydr Res. 1983 Dec 23;124(2):235–245. doi: 10.1016/0008-6215(83)88459-6. [DOI] [PubMed] [Google Scholar]
- Koga T., Okahashi N., Yamamoto T., Mizuno J., Inoue M., Hamada S. Purification and immunochemical characterization of Streptococcus sanguis ATCC 10557 serotype II carbohydrate antigen. Infect Immun. 1983 Nov;42(2):696–700. doi: 10.1128/iai.42.2.696-700.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Bush C. A., Wu S. S., Li S. C., Li Y. T., McNeil M., Tjoa S. S., Fennessey P. V. Structure of a new hexasaccharide from the coaggregation polysaccharide of Streptococcus sanguis 34. Carbohydr Res. 1987 Aug 15;166(1):133–143. doi: 10.1016/0008-6215(87)80050-2. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Barlow J. J., Matta K. L. Structural preferences of beta-galactoside-reactive lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45. Infect Immun. 1983 Aug;41(2):848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E. Inhibitors of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34: beta-galactosides, related sugars, and anionic amphipathic compounds. Infect Immun. 1982 Apr;36(1):371–378. doi: 10.1128/iai.36.1.371-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Separation of neutral oligosaccharides by high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):276–280. doi: 10.1016/0003-2697(81)90480-2. [DOI] [PubMed] [Google Scholar]
- Ogata S., Lloyd K. O. Mild alkaline borohydride treatment of glycoproteins-a method for liberating both N- and O-linked carbohydrate chains. Anal Biochem. 1982 Jan 15;119(2):351–359. doi: 10.1016/0003-2697(82)90597-8. [DOI] [PubMed] [Google Scholar]
- Rao E. V., Buchanan J. G., Baddiley J. The type-specific substance from Pneumococcus type 10A(34). Structure of the dephosphorylated repeating unit. Biochem J. 1966 Sep;100(3):801–810. doi: 10.1042/bj1000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. C., Perry M. B., Carlo D. J. The specific capsular polysaccharide of Streptococcus pneumoniae type 20. Can J Biochem Cell Biol. 1983 Apr;61(4):178–190. doi: 10.1139/o83-026. [DOI] [PubMed] [Google Scholar]
- SCHIFFMAN G., KABAT E. A., THOMPSON W. IMMUNOCHEMICAL STUDIES ON BLOOD GROUPS. XXX. CLEAVAGE OF A, B, AND H BLOOD-GROUP SUBSTANCES BY ALKALI. Biochemistry. 1964 Jan;3:113–120. doi: 10.1021/bi00889a018. [DOI] [PubMed] [Google Scholar]
- Seydel U., Lindner B., Wollenweber H. W., Rietschel E. T. Structural studies on the lipid A component of enterobacterial lipopolysaccharides by laser desorption mass spectrometry. Location of acyl groups at the lipid A backbone. Eur J Biochem. 1984 Dec 17;145(3):505–509. doi: 10.1111/j.1432-1033.1984.tb08585.x. [DOI] [PubMed] [Google Scholar]
- Spadaro A. C., Draghetta W., Del Lamma S. N., Camargo A. C., Greene L. J. A convenient manual trinitrobenzenesulfonic acid method for monitoring amino acids and peptides in chromatographic column effluents. Anal Biochem. 1979 Jul 15;96(2):317–321. doi: 10.1016/0003-2697(79)90587-6. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Hanfland P. Analysis of amino sugar-containing glycosphingolipids by combined gas-liquid chromatography and mass spectrometry. Hoppe Seylers Z Physiol Chem. 1973 Jan;354(1):21–31. doi: 10.1515/bchm2.1973.354.1.21. [DOI] [PubMed] [Google Scholar]
- Tjoa S. S., Fennessey P. V. Acylglycines. the gas chromatograph/mass spectrometric identification and interpretation of their spectra. Clin Chim Acta. 1979 Jul 2;95(1):35–45. doi: 10.1016/0009-8981(79)90334-6. [DOI] [PubMed] [Google Scholar]




