Abstract
Inefficient clearance of Aβ, caused by impaired blood-brain barrier crossing into the circulation, seems to be a major cause of Aβ accumulation in the brain of late-onset Alzheimer’s disease patients and hereditary cerebral hemorrhage with amyloidosis Dutch type. We observed association of receptor for advanced glycation end products, CD36, and low-density lipoprotein receptor (LDLR) with cerebral amyloid angiopathy in both Alzheimer’s disease and hereditary cerebral hemorrhage with amyloidosis Dutch type brains and increased low-density lipoprotein receptor-related protein-1 (LRP-1) expression by perivascular cells in cerebral amyloid angiopathy. We investigated if these Aβ receptors are involved in Aβ internalization and in Aβ-mediated cell death of human cerebrovascular cells and astrocytes. Expression of both the LRP-1 and LDLR by human brain pericytes and leptomeningeal smooth muscle cells, but not by astrocytes, increased on incubation with Aβ. Receptor-associated protein specifically inhibited Aβ-mediated up-regulation of LRP-1, but not of LDLR, and receptor-associated protein also decreased Aβ internalization and Aβ-mediated cell death. We conclude that especially LRP-1 and, to a minor extent, LDLR are involved in Aβ internalization by and Aβ-mediated cell death of cerebral perivascular cells. Although perivascular cells may adapt their Aβ internalization capacity to the levels of Aβ present, saturated LRP-1/LDLR-mediated uptake of Aβ results in degeneration of perivascular cells.
In patients with late-onset Alzheimer’s disease (AD) or hereditary cerebral hemorrhage with amyloidosis of the Dutch type (HCHWA-D), inefficient clearance of amyloid-β protein (Aβ) seems to be the key event leading to accumulation of Aβ in the brain, rather than increased Aβ production.1,2 In cerebral amyloid angiopathy (CAA), both in AD and HCHWA-D, accumulation of Aβ in the vessel walls results in degeneration of cerebrovascular cells and disruption of the blood-brain barrier.3,4,5
It has been suggested that vascular Aβ receptors, expressed by endothelial cells, transfer Aβ across the blood-brain barrier into the circulation and thus mediate clearance of Aβ from the brain.6 Alternatively, Aβ receptors may also mediate Aβ clearance via phagocytosis of Aβ by microglia and astrocytes.7,8 Both the low-density lipoprotein receptor (LDLR) and the LDLR-related protein-1 (LRP-1) may act as Aβ receptors.9,10,11 LDLR also regulates apolipoprotein E (ApoE) levels in the central nervous system and LDLR-deficient mice show increased cerebral Aβ deposition.12 LRP-1 binds both ApoE/Aβ complexes and Aβ and regulates their clearance from brain to blood.6,13 In addition, megalin (LRP2) might also regulate Aβ transport from the brain.14 Besides the LDLR family, six other potential Aβ-binding receptors have been identified. P-glycoprotein (multidrug resistance 1, MDR1) is suggested to be involved in the efflux of Aβ from the brain.15 In contrast, the receptor for advanced glycation end products (RAGE) binds and transports Aβ from blood to brain.16,17 In addition, the scavenger receptor CD36 acts as a receptor for fibrillar Aβ,18 whereas the formylpeptide receptor-like-1 (FPRL1) plays a role in the endocytosis and aggregation of Aβ in mononuclear phagocytes.19 Finally, the transmembrane amyloid precursor protein (APP) itself also functions as an Aβ receptor.20,21
Aβ is produced by neurons and, via interstitial fluid drainage,22 first encounters pericytes in capillaries and smooth muscle cells in large parenchymal and leptomeningeal vessels, before receptor-mediated trans-endothelial transport results in clearance of Aβ from brain to blood.6 This suggests that perivascular cells, next to endothelial cells, may contribute to Aβ clearance from the brain by transporting Aβ from the brain to the endothelial cells and that Aβ accumulation in CAA might result from a disturbed balance of Aβ transport from perivascular cells to endothelial cells.
To estimate the relative contribution of Aβ receptors to Aβ clearance, we investigated the distribution of Aβ receptors in AD brains and their co-localization with CAA and senile plaques in AD and HCHWA-D brains. Furthermore, we investigated the effect of Aβ on the expression levels of Aβ receptors on cultured pericytes, leptomeningeal smooth muscle cells, and astrocytes. Finally, the role of Aβ receptors in Aβ internalization and Aβ-mediated cell death of cerebrovascular cells and astrocytes was studied.
Materials and Methods
Autopsy Material
Tissue samples from the occipital cortex and hippocampus were obtained after rapid autopsy and immediately frozen in liquid nitrogen. Material was obtained from 11 AD patients (age, 82 ± 7.0 years; postmortem delay, 4.2 ± 1.0 hours), 7 of them with moderate to severe CAA and 4 control cases without neurological disease (age, 76 ± 7.7 years; postmortem delay, 4.3 ± 1.3 hours). Furthermore, tissue samples from the frontal cortex of five patients with HCHWA-D (age, 55 ± 3.3 years; postmortem delay, 3.4 ± 1.8 hours) were collected. Diagnosis and grading of AD patients were performed according to the Braak and Braak and The Consortium to Establish a Registry for Alzheimer’s Disease criteria.23,24 CAA grading was performed as described in a previous report.25 Supplemental Table 1 (see http://ajp.amjpathol.org) provides an overview of the diagnosis, Braak and Braak score, The Consortium to Establish a Registry for Alzheimer’s Disease score, CAA grade, age, postmortem interval, gender, and apolipoprotein E genotype of the patients included in this study.
Materials
Both Aβ1-40 peptide (96% pure, high pressure liquid chromatography analysis) containing the Glu22Gln mutation (D-Aβ1-40), wild-type Aβ1-42 (95% pure, high pressure liquid chromatography analysis), and wild-type Aβ1-40 (98% pure, high pressure liquid chromatography analysis) were obtained from Biosource (Etten-leur, The Netherlands). Aβ40-1 peptide (99% pure, high pressure liquid chromatography analysis) was obtained from American Peptide Company (Sunnyvale, CA). Lyophilized peptides were dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (Sigma-Aldrich, Zwijndrecht, The Netherlands), dried overnight, and subsequently dissolved in dimethyl sulfoxide, at a concentration of 5 mmol/L and stored at −80°C. Fibrillar Aβ1-42 or D-Aβ1-40 was obtained by incubating 10 μmol/L of Aβ in Eagle’s minimal essential medium (BioWhittaker Europe, Verviers, Belgium) for 3 days at 37°C and analyzed by electron microscopy; an extensive network of mature Aβ fibrils was observed in these preparations.26
Immunohistochemistry
To examine the expression pattern of Aβ receptors in control, AD, and HCHWA-D brains, serial cryosections (4 μm) were used. Sections were fixed and treated as described in previous reports.25,27 An overview of the antibodies used in this study is given in Table 1.
Table 1.
Primary Antibodies Used in This Study
| Primary antibody | Antigen | Species raised in | Dilution | Source (reference) |
|---|---|---|---|---|
| 6C6 | Aβ | Mouse | 1:2000 | Elan Pharma, San Francisco, CA |
| 100011 | CD36 | Rabbit | 1:1000 | Cayman Chemical, Ann Arbor, MI |
| Ab13177 | FPRL1 | Rabbit | 1:1000 | Abcam, Cambridge, UK |
| ST1025 | RAGE | Goat | 1:6000 | Calbiochem, Darmstadt, Germany |
| Ab10333 | MDR1 | Mouse | 1:100 | Abcam |
| H-245 | Megalin | Rabbit | 1:100 | Santa Cruz Biotechnology, Santa Cruz, CA |
| 8G1 | LRP-1 (α-chain) | Mouse | 1:100 | Progen, Heidelberg, Germany |
| 3501 | LRP-1 (β-chain) | Mouse | 1:100 | American Diagnostica Inc., Stanford, CA |
| Pab HLDL-R | LDLR | Chicken | 1:200 | Progen |
| P2–1 | APP | Rabbit | 1:1000 | Dr. W.E. Van Nostrand (28) |
Cell Culture
Human brain pericytes (HBPs), human leptomeningeal smooth muscle cells (HLSMCs), and human brain astrocytes were isolated and characterized as described previously.5,28,29,30,31 Cerebrovascular cells were maintained in Eagle’s minimal essential medium (BioWhittaker Europe) supplemented with 10% human serum (Gemini BioProducts, Calabasas, CA), 20% newborn calf serum (Life Technologies, Rockville, MD), 0.1% basic fibroblast growth factor, and 2% penicillin/streptomycin at 37°C and 5% CO2. Astrocytes were maintained in Dulbecco’s modified Eagle’s medium/HAM-F10 (1:1) containing 10% (v/v) fetal calf serum, 2 mmol/L glutamine, penicillin (100 IU/ml), and streptomycin (50 μg/ml). For degeneration studies, cells were incubated in an eight-well chamber slide (Nunc, Roskilde, Denmark) with Eagle’s minimal essential medium and 0.1% bovine serum albumin (serum-free medium) supplemented with 10 μmol/L D-Aβ1-40, 10 μmol/L wild-type Aβ1-42, or 10 μmol/L Aβ40-1,27,32 with or without 1 μmol/L receptor-activated protein (RAP) or cycloheximide (0.5 μg/ml) for 6 days. Control cells incubated with Eagle’s minimal essential medium or Dulbecco’s modified Eagle’s medium/HAM-F10 and 0.1% bovine serum albumin (serum-free medium) demonstrated normal morphology. Cell viability was quantified using a fluorescent Live/Dead Viability/Cytotoxicity kit according to the manufacturer’s description (Molecular Probes, Eugene, OR) and analyzed using a fluorescence microscope (Leica, Wetzlar, Germany). The percentage of dead cells was determined from at least four counts per well (∼800 cells per count), and experiments were performed in duplicate. Each experiment was repeated at least three times. HLSMCs incubated with scrambled sequence Aβ1-42 demonstrated neither signs of degeneration nor loss of cell viability, in line with previous data.3
Immunofluorescence Staining of Aβ Receptors on the Cell Surface
Cells cultured on eight-well chamber slides were incubated with10 μmol/L D-Aβ1-40 or 10 μmol/L wild-type Aβ1-42 for 3 days at 37°C, with or without 1 μmol/L RAP or cycloheximide (0.5 μg/ml). Cultures were washed once with phosphate-buffered saline (PBS) and then fixed with periodate-lysine-paraformaldehyde for 10 minutes. The cell preparations were incubated with monoclonal antibody 8G1 or 3501 (both anti-LRP-1) or chicken polyclonal anti-LDLR (Table 1). Subsequently, cells were incubated with Alexa Fluor 488-labeled goat anti-mouse (1:200, Molecular Probes) or biotin-labeled goat anti-chicken (Vector Laboratories, Burlingame, CA) followed by Alexa Fluor 488-labeled streptavidin (1:400, Molecular Probes). Finally, slides were incubated with Topro-3 for nuclear staining (Vector) for 45 minutes. Antibodies were diluted in PBS/0.1% bovine serum albumin, which also served as a negative control. In addition, incubation with nonspecific IgG was performed as a negative control. After each incubation, slides were extensively washed with PBS. Immunofluorescence staining was analyzed using a confocal laser-scanning microscope (Leica).
Western Blotting
Immunoblot analysis was performed with cell lysates from HBPs, HLSMCs, or astrocytes cultured in six-well plates in the presence of 10 μmol/L D-Aβ1-40, 10 μmol/L wild-type Aβ1-42, or 10 μmol/L wild-type Aβ1-40 for 3 days at 37°C, with or without 1 μmol/L RAP or cycloheximide (0.5 μg/ml). Cells were homogenized in RIPA buffer with protease inhibitors (Complete Mini; Roche, Mannheim, Germany), and equal amounts of protein were loaded and electrophoresed on 15% polyacrylamide gels. Nonspecific protein binding was blocked by preincubation with Odyssey-blocking buffer (according to the manufacture’s guidelines; LI-COR, Bad Homburg, Germany). Bound anti-LRP (8G1) or anti-LDLR (chicken) was detected using Alexa Fluor 680- or 800-labeled goat anti-mouse, or biotin-labeled goat anti-chicken followed by Alexa Fluor 680-labeled streptavidin (Molecular Probes). All immunoblots were performed at least three times, and a representative blot is shown in the Results section. Western blot analysis and quantification was performed using the Odyssey infrared imaging system (LI-COR).
Quantitative Immunofluorescence Staining
A quantitative immunofluorescence staining assay with infrared detection using the Odyssey infrared imaging system was performed as described.33,34 In short, cerebrovascular cells and astrocytes (20,000 cells/well) were cultured in fibronectin-coated 96-well plates (Nunc), for 1 to 2 days until confluence. Additionally, the cells were incubated with serum-free medium for at least 4 hours. Next, fresh medium containing 0.1% bovine serum albumin with or without 1 to 10 μmol/L D-Aβ1-40, 1 to 10 μmol/L wild-type Aβ1-42,1 μmol/L RAP, or cycloheximide (0.5 μg/ml) was added to the cells. On incubation for 3 days at 37°C, cells were rinsed twice with PBS and fixed with 4% paraformaldehyde for 20 minutes at room temperature. Cells were then washed with either PBS (to quantify cell surface immunoreactivity only) or PBS containing 0.1% Triton X-100 (to quantify overall cellular immunoreactivity) and rinsed again before blocking with 150 μl of LI-COR Odyssey blocking buffer (1:1 in PBS) for 90 minutes at room temperature. Primary antibodies [anti-Aβ (40-4) and anti-LRP-1 (8G1)] were diluted in Odyssey blocking buffer, and cells were incubated with the diluted antibodies (50 μl/well) overnight at 4°C. Cells were repeatedly washed with PBS or PBS-0.05% Triton X-100 (PBS-T) and incubated with secondary antibodies diluted in Odyssey blocking buffer at room temperature for 1 hour; ie, Alexa Fluor 680-labeled goat anti-mouse, IRDye 800CW-labeled goat anti-rabbit (1:400; Rockland Immunochemicals, Gilbertsville, PA) or biotin-labeled secondary antibody goat anti-chicken (1:200, Vector). Cells were again rinsed with PBS and PBS-T and, in case of the secondary anti-chicken antibody, incubated with Alexa Fluor 680-labeled streptavidin for another 60 minutes at room temperature. Analysis was performed using the Odyssey infrared imaging system. The intracellular levels of immunoreactivity were determined by subtracting the signal obtained in cells, treated with PBS only, from those treated with PBS containing 0.1% Triton X-100.
Results
Expression of Aβ Receptors in Control, AD, and HCHWA-D Brains
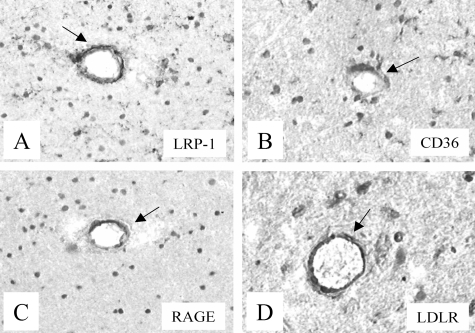
Patterns and levels of expression of Aβ receptors in vessels in which no Aβ had accumulated were similar in AD, HCHWA-D, and control brains. No Aβ was detected (mAb 6C6) in brain vessels of control brains. Immunoreactivity for RAGE was observed in astrocytes of white and gray matter and in medium-sized parenchymal vessels (diameter, 75 to 150 μm) (Figure 1C), whereas no staining in leptomeningeal vessels and capillaries was seen (Table 2). LRP-1 immunostaining was observed in astrocytes of the white matter, in neurons, and in both leptomeningeal vessels and medium-sized parenchymal (Figure 1A) vessels in control brains. Expression of MDR1 was observed in capillaries (data not shown). APP, as well as CD36 (Figure 1B), immunostaining was observed in astrocytes and both leptomeningeal and medium-sized parenchymal vessels (Table 2). Anti-APP staining was also present in neurons (data not shown). LDLR immunostaining was observed in astrocytes, neurons, and capillaries and was also present in leptomeningeal and medium-sized parenchymal vessels in control brains (Figure 1D). Both anti-FPRL1 and anti-megalin staining was demonstrated in astrocytes but was absent in leptomeningeal and medium-sized parenchymal vessels (Table 2), and anti-FPRL1 immunoreactivity was observed in neurons.
Figure 1.
Immunohistochemical staining of Aβ receptor antibodies in neocortex of control brains. Both anti-LRP-1 (A, arrow) and anti-RAGE (C, arrow) antibodies demonstrated immunoreactivity in normal medium-sized parenchymal vessels in control brains. Both anti-CD36 (B, arrow) and anti-LDLR (D, arrow) antibodies were immunoreactive in medium-sized parenchymal vessels in control brain. Original magnifications, ×250.
Table 2.
Overview of the Expression of Aβ Receptors in Vessels of Control, AD, and HCHWA-D Brains, and Their Association with CAA and SPs in These Brains
| Vessels in control, AD, and HCHWA-D brains
|
CAA Leptomeningeal and parenchymal vessels | SPs Classic | Diffuse | ||||
|---|---|---|---|---|---|---|---|
| Leptomeningeal vessels | Medium-sized parenchymal vessels | Capillaries | Astrocytes | ||||
| APP | + | + | − | + | − | + | − |
| MDR1 | − | + | + | − | − | − | − |
| RAGE | − | + | − | + | + | − | − |
| LRP-1 | + | + | − | + | + | + | − |
| Megalin | − | − | − | + | − | + | − |
| CD36 | + | + | − | + | + | + | − |
| FPRL1 | − | − | − | + | − | + | − |
| LDLR | + | + | + | + | + | − | − |
Expression of Aβ receptors in various brain vessels and in cerebral amyloid angiopathy (CAA) and diffuse senile plaques (SPs) in Alzheimer’s disease (AD) and hereditary cerebral hemorrhage with amyloidosis of the Dutch type (HCHWA-D). The presence of Aβ receptors staining in a specific vessel or lesion is indicated as follows: absent (−), present (+).
Expression of Aβ Receptors in CAA and SPs in AD and HCHWA-D Brains
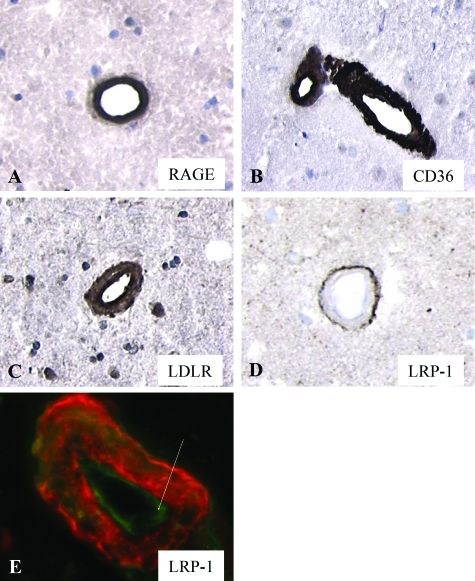
In both AD and HCHWA-D brains, Aβ-affected vessels were identified by their intense staining by the anti-Aβ antibody (mAb 6C6) (not shown) and demonstrated thickening of the vessel wall, a general feature of CAA. Anti-RAGE (Figure 2A), anti-CD36 (Figure 2B), and anti-LDLR (Figure 2C) antibodies stained CAA of leptomeningeal and medium-sized parenchymal vessels of both AD and HCHWA-D brains. Anti-LRP-1 immunoreactivity was observed at the abluminal side of CAA-affected vessels (Figure 2D), suggestive of increased expression by perivascular cells, and in endothelial cells (Figure 2E), in both leptomeningeal and medium-sized parenchymal vessels. No immunostaining for APP, MDR1, megalin, and FPRL1 was observed in CAA.
Figure 2.
Immunohistochemical staining of Aβ receptor antibodies in CAA in the neocortex of AD brains. RAGE (A), CD36 (B), and LDLR (C) immunostaining was observed in CAA, whereas LRP-1 expression was increased in perivascular cells but not in CAA itself (D). E: Immunofluorescent staining of LRP-1 (green) was also observed in endothelial cells in CAA vessels (Aβ stained red). Original magnifications: ×250 (A–D); ×400 (E).
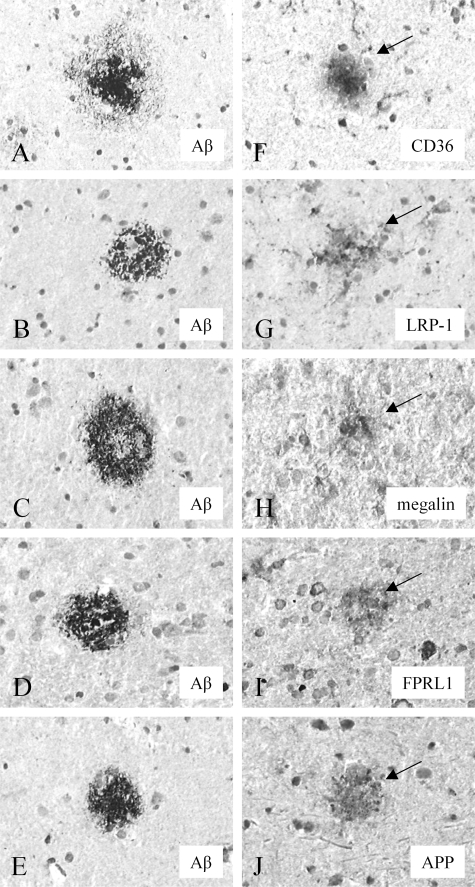
The anti-Aβ antibody (mAb 6C6) stained both classic and diffuse SPs (Figure 3, A–E) in AD brains and diffuse SPs in HCHWA-D brains. A comparison with the staining of the Aβ receptor antibodies in serial sections demonstrated co-localization of CD36 (Figure 3F), LRP-1 (Figure 3G), megalin (Figure 3H), FPRL1 (Figure 3I), and APP (Figure 3J) immunostaining with classic SPs in AD brains, but not with diffuse SPs in AD and HCHWA-D brains. No immunostaining for RAGE, MDR1, and LDLR was observed in both classic and diffuse SPs in AD and diffuse SPs in HCHWA-D brains. No staining was observed using nonspecific IgG as a negative control. The results of the immunohistochemical stainings for the Aβ receptors in normal, AD, and HCHWA-D brains are summarized in Table 2. Because we observed a close association of LRP-1, LDLR, RAGE, and CD36 with CAA, we investigated the effect of Aβ on the levels of expression of these Aβ receptors by cerebrovascular cells and astrocytes.
Figure 3.
Immunohistochemical staining of Aβ receptor antibodies in classic SPs in the neocortex of AD brain. The anti-Aβ (mAb 6C6) antibody stained both classic and diffuse SPs in AD brains (A–E). CD36 (F, arrow), LRP-1 (G, arrow), megalin (H, arrow), FPRL1 (I, arrow), and APP (J, arrow) immunostaining was observed in classic SPs. Serial sections: A, F; B, G; C, H; D, I; E, J. Original magnifications, ×250.
Aβ-Mediated Up-Regulation of Aβ Receptors by Cerebrovascular Cells
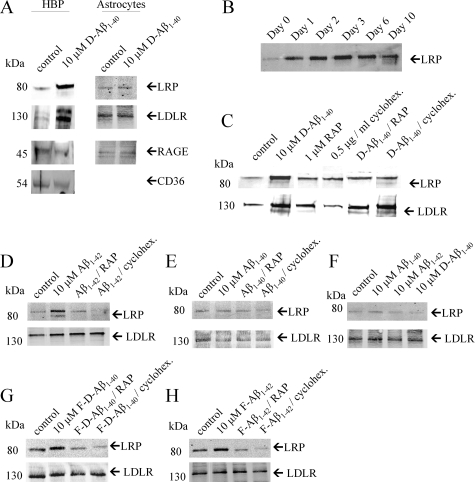
We analyzed expression of the Aβ receptors in cultured HBPs, HLSMCs, and astrocytes. LRP-1, LDLR, RAGE, and CD36 were observed in both HBPs and HLSMCs using Western blot analysis (Figure 4A). In astrocytes, however, LRP-1, LDLR, and RAGE were observed, whereas expression of CD36 was absent (Figure 4A). LRP-1 and LDLR expression, but not that of CD36 or RAGE, was increased in HBPs and HLSMCs incubated with 10 μmol/L D-Aβ1-40 for 3 days compared to the control situation (Figure 4A), whereas similar incubations in astrocytes did not result in up-regulation of any of the Aβ receptors studied (Figure 4A). Several independent incubations of HBPs with 10 μmol/L D-Aβ1-40 for 3 days demonstrated an increase of both LRP-1 and LDLR by an average factor of 3.1 and 1.9, respectively, compared to control levels. In addition, up-regulation of LRP-1 in HBPs was sustained until 10 days after treatment with 10 μmol/L D-Aβ1-40 (Figure 4B).
Figure 4.
Western blot analysis of LRP-1 and LDLR expression in cultured HBPs and astrocytes. A: HBPs and astrocytes were incubated with or without 10 μmol/L D-Aβ1-40 for 3 days at 37°C. In HBPs, expression of LRP-1, LDLR, RAGE, and CD36 was observed, and both LRP-1 and LDLR were up-regulated by incubation with 10 μmol/L D-Aβ1-40. In astrocytes expression of LRP-1, LDLR, and RAGE was observed, but Aβ did not affect receptor expression. B: Treatment of HBPs with 10 μmol/L D-Aβ1-40 resulted in LRP-1 up-regulation after 1 day and sustained until 10 days after treatment with 10 μmol/L D-Aβ1-40. C: In HBPs co-incubated with RAP (1 μmol/L) or cycloheximide (0.5 μg/ml) for 3 days at 37°C, reduction of LRP-1 up-regulation was observed, whereas LDLR expression remained unaffected. D: Similar effects on both LRP-1 and LDLR expression on HBPs were observed after incubations with Aβ1-42. E: Incubation of HBPs with Aβ1-40 had no effect on both LRP-1 and LDLR expression. F: Astrocytes incubated with 10 μmol/L of Aβ1-40, Aβ1-42, or D-Aβ1-40, demonstrated no effects on both LRP-1 and LDLR expression. G and H: Incubation of either 10 μmol/L fibrillar D-Aβ1-40 (G) or Aβ1-42 (H) resulted in increased LRP-1 expression by HBPs, whereas no differences were observed for LDLR levels. In addition, co-incubation of fibrillar Aβ with RAP or cycloheximide inhibited this effect.
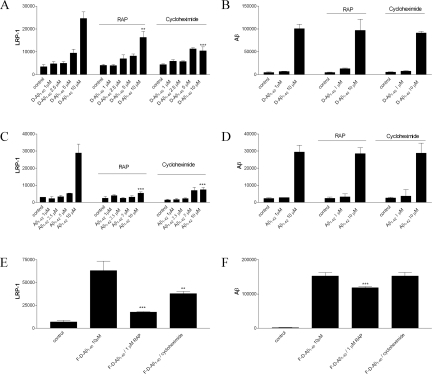
To antagonize both LRP-1 and LDLR up-regulation in HBPs, cells were co-incubated with RAP (1 μmol/L) or cycloheximide (0.5 μg/ml).35,36 Treatment of HBPs for 3 days with RAP or cycloheximide alone had no effect on both LRP-1 and LDLR expression, whereas co-incubation of 10 μmol/L D-Aβ1-40 with RAP or cycloheximide reduced LRP-1 expression to control levels but did not affect LDLR expression (Figure 4C). Incubation of HBPs with 10 μmol/L Aβ1-42 resulted in up-regulation of LRP-1 expression but not of LDLR (Figure 4D). Co-incubation with 10 μmol/L Aβ1-42 and RAP or cycloheximide again reduced LRP-1 expression to control levels, whereas expression of LDLR remained unaffected (Figure 4D). Aβ1-40 had no effect on LRP-1 or LDLR expression and, consequently, co-incubation with RAP or cycloheximide remained ineffective (Figure 4E). In astrocytes incubated with Aβ1-40, Aβ1-42, or D-Aβ1-40, no effects on both LRP-1 and LDLR expression were observed (Figure 4F). Treatment of HBPs with either 10 μmol/L fibrillar D-Aβ1-40 (Figure 4G) or fibrillar Aβ1-42 (Figure 4H) also resulted in an increase in LRP-1 expression by HBPs (average factor of 1.6 and 1.3, respectively), whereas no differences were found for LDLR levels (Figure 4G). Co-incubation of fibrillar D-Aβ1-40 (Figure 4G) or fibrillar Aβ1-42 (Figure 4H) with either RAP or cycloheximide reduced the LRP-1 up-regulation by fibrillar Aβ.
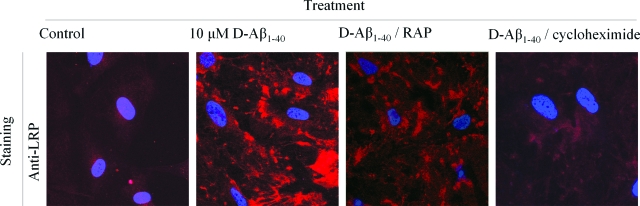
The effects of D-Aβ1-40 on LRP-1 expression in HBPs were confirmed by confocal laser-scanning microscopy (Figure 5). In untreated cells, LRP-1 demonstrated a low cytoplasmic expression level. After incubation with 10 μmol/L D-Aβ1-40 for 3 days at 37°C, the overall anti-LRP-1 immunoreactivity was increased throughout the cytoplasm, whereas co-incubations with RAP or cycloheximide decreased anti-LRP-1 immunoreactivity compared to D-Aβ1-40 alone (Figure 5). Taken together, in contrast to LDLR, LRP-1 up-regulation by Aβ is inhibited using RAP and cycloheximide. This suggests a different mechanism of receptor induction by Aβ. To investigate further the relation between Aβ and LRP-1 expression, LRP-1 expression was studied in more detail by quantitative immunofluorescence. Treatment of HBPs with 1 to 10 μmol/L D-Aβ1-40 resulted in a dose-dependent increase in LRP-1 expression that was antagonized by RAP as well as by cycloheximide (Figure 6A). Neither RAP nor cycloheximide affected the association of D-Aβ1-40 with the cell surface (Figure 6B). Similar to D-Aβ1-40, 1 to 10 μmol/L Aβ1-42 also induced a dose-dependent increase in LRP-1 expression by HBPs (Figure 6C), and co-incubation with RAP or cycloheximide inhibited this increase (Figure 6C). As observed for D-Aβ1-40, both RAP and cycloheximide did not affect Aβ1-42 immunoreactivity on the cell surface (Figure 6D). We observed similar effects on LRP-1 expression and anti-Aβ immunoreactivity when cultured HLSMCs were used (not shown). Incubation of fibrillar D-Aβ1-40 or Aβ1-42 (not shown) resulted in increased expression of LRP-1 by HBPs, which could be inhibited both by RAP (∼68% reduction) and cycloheximide (∼52% reduction) (Figure 6E). Co-incubation of fibrillar D-Aβ1-40 or Aβ1-42 (not shown) with RAP resulted in a slightly, but significantly, decreased (∼20%) anti-Aβ immunoreactivity on the cells, whereas incubation of cycloheximide had no effect (Figure 6F). Thus, although RAP inhibits Aβ-mediated LRP-1 induction, it is not capable of preventing Aβ accumulation at the cell surface.
Figure 5.
Confocal laser-scanning microscopy analysis of Aβ-mediated up-regulation of LRP-1 in HBPs. HBPs incubated with 10 μmol/L D-Aβ1-40, or in combination with RAP (1 μmol/L) or cycloheximide (0.5 μg/ml) for 3 days at 37°C. Increased immunoreactivity of anti-LRP antibody (red) was observed after treatment with 10 μmol/L D-Aβ1-40, compared to control levels of LRP-1 in HBPs. Co-incubation of D-Aβ1-40 with RAP or cycloheximide demonstrated a reduced immunoreactivity of the anti-LRP-1 antibody. Nuclei are counterstained blue. Original magnifications, ×630.
Figure 6.
Quantitative immunofluorescence analysis of LRP-1 expression in HBPs. HBPs were incubated with peptide concentrations as indicated for 3 days at 37°C, and anti-LRP-1 and anti-Aβ immunoreactivity were analyzed as described in Materials and Methods. A: Treatment with 1 to 10 μmol/L D-Aβ1-40 resulted in a dose-dependent increase in LRP-1 expression. This increased expression was antagonized by co-incubations with RAP (1 μmol/L) or cycloheximide (0.5 μg/ml). B: Co-incubations of D-Aβ1-40 with both RAP and cycloheximide had no effect on cell surface Aβ compared to D-Aβ1-40 alone. C and D: Similar effects were also observed in treatment with 1 to 10 μmol/L Aβ1-42 and in co-incubations of Aβ1-42 with RAP or cycloheximide. E: Treatment of HBPs with 10 μmol/L of fibrillar D-Aβ1-40 (F-D-Aβ1-40) resulted in increased LRP-1 expression, whereas co-incubation of fibrillar D-Aβ1-40 with either RAP or cycloheximide reduced this effect. F: In addition, co-incubation of 10 μmol/L fibrillar D-Aβ1-40 with RAP resulted in moderately decreased D-Aβ1-40 accumulation at the cell surface, compared to D-Aβ1-40 alone, whereas co-incubations with cycloheximide had no effect on accumulation of D-Aβ1-40 at the cell surface. Statistical analysis was performed using Student’s t-test. The level of significance of the difference with 10 μmol/L (F-) D-Aβ1-40 or Aβ1-42 and the combination with RAP or cycloheximide is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, P > 0.05 is not indicated. Mean ± SD of quadruplicates are shown.
LRP Is Involved in Aβ Internalization and Aβ-Mediated Cell Death of Cerebrovascular Cells
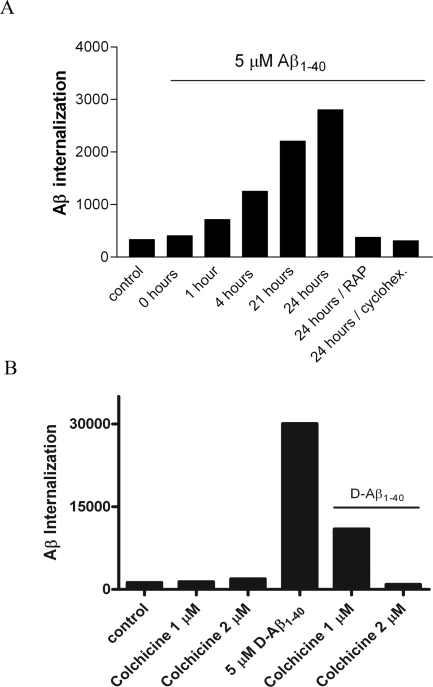
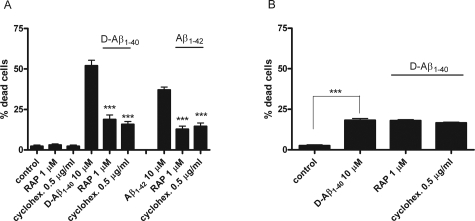
To analyze the role of LRP-1 in Aβ internalization by HBPs, quantitative immunofluorescence of Aβ internalized by the cells was performed. HBPs were incubated with 5 μmol/L Aβ1-40 for 0, 1, 4, 21, and 24 hours at 37°C. Increasing levels of intracellular Aβ were observed throughout time (Figure 7A). Co-incubation of 5 μmol/L Aβ1-40 with either RAP or cycloheximide inhibited internalization of Aβ (Figure 7A). Similar effects on internalization were observed using 5 μmol/L D-Aβ1-40 (data not shown). Internalization of 5 μmol/L D-Aβ1-40 was completely blocked by co-incubation with the endocytosis inhibitor,37 colchicine (2 μmol/L) (Figure 7B). Astrocytes incubated with 5 μmol/L Aβ1-40 for 0, 1, 4, and 24 hours at 37°C, also demonstrated internalization of Aβ but co-incubations with either RAP or cycloheximide were ineffective in preventing Aβ internalization (not shown). Thus, Aβ internalization by cerebrovascular cells is mediated by LRP-1, whereas Aβ internalization in astrocytes is mediated via a different mechanism possibly involving a different receptor(s). To investigate the role of Aβ internalization in Aβ-mediated cerebrovascular cell death, we incubated cerebrovascular cells with Aβ and LRP-1 antagonists and quantified cell viability. Incubation of cultured HBPs with 10 μmol/L D-Aβ1-40 for 6 days reduced the percentage of viable cells to 51%, whereas in control incubations 4% of the cells were dead (Figure 8A). Aβ treatment resulted in visible signs of cellular degeneration, with cell contours becoming blurred, although all cell bodies remained attached to the culture dish. RAP and cycloheximide had no effect on cell viability (Figure 8A). Co-incubation of 10 μmol/L D-Aβ1-40 with RAP reduced the percentage of dead cells to 21%, whereas co-incubation of cycloheximide reduced cell death to 18% (Figure 8A). After 6 days, the percentage of dead cells in cultured HBPs treated with 10 μmol/L Aβ1-42 was 42%, whereas after co-incubation with RAP (17%) or cycloheximide (18%) cell death was similar to incubations of D-Aβ1-40 with RAP or cycloheximide (Figure 8A). Treatment with 10 μmol/L Aβ1-40 did not affect cell viability. Incubation with 25 μmol/L of the inverted wild-type sequence Aβ40-1 had no effect on cell death, compared to the control incubations (not shown).26 Incubation of cultured astrocytes with 10 μmol/L D-Aβ1-40 for 6 days resulted in 18% cell death, whereas in controls 4% cell death was observed (Figure 8B). Co-incubation of 10 μmol/L D-Aβ1-40 with RAP or cycloheximide resulted in approximately similar percentages of dead cells compared to D-Aβ1-40 alone (Figure 8B).
Figure 7.
Internalization of Aβ by HBPs. HBPs were incubated with 5 μmol/L Aβ1-40 (A) or D-Aβ1-40 (B), with or without RAP (1 μmol/L) or cycloheximide (0.5 μg/ml) for 0, 1, 4, 21, and 24 hours at 37°C, or colchicine (1 or 2 μmol/L) for 2 days at 37°C. Aβ immunoreactivity was analyzed, as described in Materials and Methods. Cell surface immunoreactivity was subtracted from overall immunoreactivity resulting in the percentage of Aβ that is internalized. A: Increasing levels of Aβ1-40 were observed in time, whereas co-incubation of 5 μmol/L Aβ1-40 with RAP or cycloheximide inhibited Aβ internalization after 24 hours, compared to Aβ1-40 alone. B: Co-incubation of D-Aβ1-40 with colchicine (2 μmol/L) completely blocked D-Aβ1-40 internalization by HBPs.
Figure 8.
LRP antagonists reduce Aβ-mediated cell death of cerebrovascular cells. Effects of RAP or cycloheximide on cerebrovascular and astrocyte cell death after incubation with 10 μmol/L D-Aβ1-40, 10 μmol/L Aβ1-42, with or without RAP (1 μmol/L) or cycloheximide (0.5 μg/ml) for 6 days at 37°C. A: Incubation of both 10 μmol/L D-Aβ1-40 or 10 μmol/L Aβ1-42 resulted in cell death of HBPs, ∼51% and 43%, respectively. Co-incubations with either RAP or cycloheximide significantly reduced both D-Aβ1-40 and Aβ1-42-mediated cell death of HBPs. B: Astrocytes incubated with 10 μmol/L D-Aβ1-40 showed a cell death of ∼18%, whereas co-incubation with RAP or cycloheximide had no effect on cell viability compared to D-Aβ1-40 alone. Statistical analysis was performed using Student’s t-test. The level of significance of the difference with 10 μmol/L D-Aβ1-40 or Aβ1-42 and combinations with RAP or cycloheximide is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, P > 0.05 is not indicated. Mean ± SD are shown.
Discussion
The main findings of our study are that 1) expression of LRP-1, LDLR, RAGE, and CD36 are associated with CAA in both AD and HCHWA-D brains; 2) Aβ increases LRP-1 and LDLR expression by cerebrovascular cells in vitro; 3) RAP inhibits the Aβ-mediated increased LRP-1 expression, but not that of LDLR; and 4) both internalization of Aβ and Aβ-mediated cell death can be inhibited by RAP (summarized in Table 3).
Table 3.
Summary of the Data Presented in This Study
| HBPs/HLSMCs
|
Astrocytes
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LRP-1LDLR
|
LRP-1LDLR
|
|||||||||||||||||
| Effects on receptor expression
|
Aβ accumulation on cell surface
|
Effects on cell viability
|
Internalization
|
Effects on receptor expression | Effects on cell viability
|
Internalization
|
||||||||||||
| − | RAP | CH | − | RAP | CH | RAP | CH | RAP | CH | RAP | CH | RAP | CH | RAP | CH | |||
| Addition: | ||||||||||||||||||
| D-Aβ1–40 | h | i | i | h | − | − | − | − | h | h | i | i | − | − | − | − | ||
| Aβ1–40 | − | − | − | − | i | i | - | - | − | − | ||||||||
| Aβ1–42 | h | i | i | − | − | − | - | - | h | h | − | − | − | − | ||||
| F-D-Aβ1–40 | h | i | i | − | − | − | i | − | h | − | − | − | ||||||
| F-Aβ1–42 | h | i | i | − | h | − | − | − | ||||||||||
HBPs, human brain pericytes; HLSMCs, human leptomenigeal smooth muscle cells; LRP-1, low-density lipoprotein receptor-related protein-1; LDLR, low-density lipoprotein receptor; D-Aβ1–40, amyloid-beta1–40 containing the Glu22Gln mutation; F-D-Aβ1–40, fibrillar D-Aβ1–40; RAP, receptor-associated protein; CH, cycloheximide; h, increased; i, inhibited; −, no effect.
We observed vascular expression of a number of potential Aβ receptors (LRP-1, RAGE, CD36, LDLR, APP) in control and AD and HCHWA-D brains. LRP-1 and CD36 were only observed in the larger brain vessels, whereas MDR1 was predominantly found in capillaries. These findings suggest that transvascular transport of Aβ might be mediated by different Aβ receptors in different types of brain vessels. Well known Aβ receptors such as LRP-1 and RAGE were predominantly observed in middle-sized parenchymal vessels, suggesting that, apart from the capillaries, the contribution of these larger vessels to Aβ transport across the blood-brain barrier may be substantial. However, how the distribution pattern of the various Aβ receptors reflects Aβ transport in vivo remains to be elucidated.
From several in vitro studies and observations in transgenic mouse models it was suggested that in AD brains expression of Aβ receptors such as LRP-1 might be decreased and that this change might be directly related to the accumulation of Aβ in AD brains.13,16,38,39 In contrast to these findings, we did not observe spatial and quantitative differences in expression of LRP-1 or of other potential Aβ receptors in brain vessels between control, AD, and HCHWA-D brains. Nevertheless, because accumulation of Aβ in AD is a long-term chronic process, subtle differences in expression of LRP-1 or other Aβ receptors that remain undetected by immunohistochemical analysis may be significant for the process of Aβ clearance.
Aβ is cleared from the brain via several clearance mechanisms, such as uptake and degradation by astrocytes and microglia,7,8 via Aβ-degrading enzymes, such as neprilysin, endothelin-converting enzyme, and insulin-degrading enzyme,40,41,42 and via transport by cerebral cells across the blood-brain barrier into the blood. In brain, Aβ is predominantly produced by neurons and transported toward the cerebral vasculature by interstitial fluid drainage.22 In several studies it has been demonstrated that transport from brain to blood is mediated by LRP-1 and vice versa by RAGE.6,13,16,43 In normal brain, clearance of Aβ is the net result of these counteractive mechanisms. Trans-endothelial LRP-1-mediated transport of Aβ seems to be an essential part of this process. In AD, endothelial LRP-1 expression seems to be reduced leading to impaired export of Aβ from the brain.43 However, because Aβ is a constituent of the interstitial fluid flowing along perivascular cells toward the cerebrospinal fluid and because Aβ in CAA is initially deposited in the adventitia,44 it is very possible that perivascular cells (ie, pericytes and smooth muscle cells) contribute to the process of transport of Aβ from brain to blood as well. The deposition of Aβ might be a direct consequence of a combination of increased cerebral production followed by LRP-1-mediated perivascular accumulation and impaired export of Aβ by endothelial cells to the blood. However, although we observed internalization of Aβ by perivascular cells, both the cellular fate of Aβ after internalization by these cells and the transport route of Aβ through the cell’s interior or via the membrane of these cells remains to be determined.
Although immunohistochemical studies do not allow for exact assignment of receptor expression to pericytes, particularly because of the small size of pericytes,29 our in vitro data demonstrated that both pericytes and SMCs express LRP-1, LDLR, RAGE, and CD36. Additionally, expression of LRP-1 and LDLR by these cerebrovascular cells was increased in response to Aβ, suggesting that the capacity to internalize Aβ is positively influenced by the Aβ levels present. This, however, is in contrast to published observations, describing down-regulated endothelial LRP-1 expression in response to Aβ treatment.43 Besides, both Aβ1-42 and D-Aβ1-40 induced LRP-1 and LDLR expression, yet only LRP-1 expression was inhibited by RAP. Furthermore, we described that both cultured HBPs and SMCs internalize Aβ, which can also be inhibited by RAP. These data suggest that predominantly LRP-1 mediates uptake of Aβ,45 although we cannot exclude the possible role of LDLR because 1 μmol/L RAP antagonizes both LRP-1 and LDLR.36 In conclusion, both pericytes and SMCs may contribute to Aβ clearance from the brain, a function that, given their anatomical position and contact with the interstitial fluid, seems to be relevant in vivo.
Thus, although pericytes and SMCs may contribute to vascular clearance of Aβ, it is conceivable that at relatively high concentrations of Aβ the cells are unable to remove this Aβ by either degradation or secretion. Because LRP-1 and LDLR continuously circulate between the cell surface and cytoplasmic vesicles, a shift from cytoplasmic to cell surface expression of these receptors may precede the up-regulation induced by only high levels of Aβ.46 Furthermore, both LRP-1 and LDLR may serve as an anchor for Aβ at the cell surface, although we observed no effects of RAP on Aβ accumulation at the cell surface. However, this effect was probably attributable to an excess availability of Aβ or by Aβ binding other receptors or proteins that function as its anchor on the cell surface, such as APP. Thus, the saturated uptake of Aβ, leading to the accumulation of Aβ at the cell surface, may subsequently result in degeneration of cerebrovascular cells, leading to CAA. Evidence that this saturated uptake is, at least partly, mediated by LRP-1 comes from the observation that 1 μmol/L RAP antagonizes LRP-1 expression (this study)35,36 and inhibits Aβ-mediated cell death (this study).
Accumulation of Aβ at the cell surface of cerebrovascular cells is tightly linked to degeneration of these cells.4,5,47 However, in this study both RAP and cycloheximide inhibited the monomeric as well as the fibrillar Aβ-mediated increase in expression of LRP-1 and reduced monomeric and fibrillar Aβ-mediated cell death, without affecting Aβ accumulation at the cell surface. These data are in line with a single report describing a similar uncoupling of Aβ cell surface association and cell death after co-incubation with humanin.48 Thus, our study suggests that rather than the association of Aβ with the cell surface, resulting in disturbance of the cell membrane integrity, downstream cellular signaling events, possibly mediated by LRP-1 or, to a minor extent, LDLR, are crucial for initiating cellular degeneration. This is also supported by our observations that D-Aβ1-40 and Aβ1-42 induced both LRP-1 up-regulation and cerebrovascular cell death, in contrast to Aβ1-40, which demonstrates no effect on LRP-1 expression and on cell viability.
In contrast to the results with pericytes, LRP-1 and LDLR expression by astrocytes that, to some degree, may also contribute to cerebral Aβ clearance,7 were not increased in response to Aβ treatment. Thus, although astrocytes may internalize Aβ, their capacity to do so is not increased in the same way as observed for HBPs and HLSMCs. Furthermore, both internalization of Aβ and degeneration of these cells were not inhibited by RAP, which suggests that receptor stability in astrocytes of both LRP-1 or LDLR differs from cerebrovascular cells or that Aβ receptors other than LRP-1 or LDLR, or receptor-independent mechanisms are involved in the internalization of Aβ by astrocytes. Thus, the exact role of these Aβ receptors in Aβ internalization and Aβ-mediated cell death toward astrocytes remains to be elucidated.
Pericyte LRP-1 expression was up-regulated by Aβ1-42, D-Aβ1-40, and fibrillar forms of these Aβ peptides, but not by the less amyloidogenic Aβ1-40. In addition, Aβ1-42 did not result in up-regulation of LDLR, as observed for D-Aβ1-40. It has been demonstrated that LRP-1 binds with higher affinity to Aβ1-40 than Aβ1-42 and D-Aβ1-40, which suggests that receptor-mediated clearance of Aβ1-40 is more efficient than for other Aβ isoforms and which may be an explanation for both the lack of LRP-1 up-regulation by Aβ1-40 and the absence of Aβ1-40-mediated cell death of HBPs and HLSMCs.43 In HCHWA-D patients, a mutation in the Aβ1-40 sequence results in the more amyloidogenic D-Aβ1-40. This Aβ isoform is cleared less efficiently,43 and possibly as a compensation for this reduced efficiency, LRP-1 and LDLR expression is up-regulated by perivascular cells. Both the amyloidogenic properties and the endothelial clearance of Aβ1-42 are intermediate compared to Aβ1-40 and D-Aβ1-40, which may accordingly be compensated for by increased LRP-1 expression only. D-Aβ1-40 induced a more extensive degeneration of cerebrovascular cells than Aβ1-42, which may be related to the extensive development of CAA in HCHWA-D patients,4,49 possibly as a result of saturated uptake by perivascular cells.
Our data suggest that cerebrovascular cell death induced by Aβ might be a receptor-mediated process instead of a nonspecific loss of membrane integrity and that an excess of Aβ at the cell surface leads to binding to cell surface compounds other than LRP-1/LDLR. In this situation, the number of Aβ receptors is insufficient to achieve complete internalization of Aβ. In contrast, internalization and cell death induced by Aβ in astrocytes might be principally regulated by other receptors or by yet other mechanisms. We suggest that expression of Aβ receptors by pericytes and SMCs may contribute to the transport of Aβ from brain to blood and that these cells may adapt their transport capacity to the levels of Aβ present. If the cellular Aβ levels become saturated because of either high concentrations of Aβ or the presence of relatively amyloidogenic forms of Aβ (eg, D-Aβ1-40, Aβ1-42), Aβ will accumulate in vessel walls resulting in degeneration of cerebrovascular cells, and CAA will develop. However, the relative contributions of endothelial cells on the one hand and pericytes and SMCs on the other, in the process of Aβ clearance from brain to blood and the development and progression of CAA, requires further study.
Supplementary Material
Acknowledgments
We thank Dr. D. Schenk and Dr. W.E. Van Nostrand for their generous gifts of antibodies.
Footnotes
Address reprint requests to Dr. M.M. Verbeek, Department of Neurology, 830 LKN, Radboud University Nijmegen Medical Centre, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands. E-mail: m.verbeek@cukz.umcn.nl.
Supported by the Internationale Stichting Alzheimer Onderzoek (grants 01506 and 03517), Zon-MW Innovational Research (grant 917.46.331, Vidi program), the Hersenstichting Nederland (grants 11F03(2)11 and 14F06.18), and the National Institutes of Health (grant AG027924 to G.B.).
M.M.M.W. and I.O.-H. contributed equally to this study.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32:177–180. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Ghiso J, Frangione B. Amyloidosis and Alzheimer’s disease. Adv Drug Deliv Rev. 2002;54:1539–1551. doi: 10.1016/s0169-409x(02)00149-7. [DOI] [PubMed] [Google Scholar]
- Davis-Salinas J, Saporito-Irwin SM, Cotman CW, Van Nostrand WE. Amyloid beta-protein induces its own production in cultured degenerating cerebrovascular smooth muscle cells. J Neurochem. 1995;65:931–934. doi: 10.1046/j.1471-4159.1995.65020931.x. [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Melchor JP, Ruffini L. Pathologic amyloid beta-protein cell surface fibril assembly on cultured human cerebrovascular smooth muscle cells. J Neurochem. 1998;70:216–223. doi: 10.1046/j.1471-4159.1998.70010216.x. [DOI] [PubMed] [Google Scholar]
- Verbeek MM, de Waal RM, Schipper JJ, Van Nostrand WE. Rapid degeneration of cultured human brain pericytes by amyloid beta protein. J Neurochem. 1997;68:1135–1141. doi: 10.1046/j.1471-4159.1997.68031135.x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Clearing amyloid through the blood-brain barrier. J Neurochem. 2004;89:807–811. doi: 10.1111/j.1471-4159.2004.02385.x. [DOI] [PubMed] [Google Scholar]
- Nagele RG, D’Andrea MR, Lee H, Venkataraman V, Wang HY. Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971:197–209. doi: 10.1016/s0006-8993(03)02361-8. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Schlosshauer B. The blood-brain barrier: morphology, molecules, and neurothelin. Bioessays. 1993;15:341–346. doi: 10.1002/bies.950150508. [DOI] [PubMed] [Google Scholar]
- Arélin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT. LRP and senile plaques in Alzheimer’s disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002;104:38–46. doi: 10.1016/s0169-328x(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Fryer JD, DeMattos RB, McCormick LM, O’Dell MA, Spinner ML, Bales KR, Paul SM, Sullivan PM, Parsadanian M, Bu G, Holtzman DM. The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J Biol Chem. 2005;280:25754–25759. doi: 10.1074/jbc.M502143200. [DOI] [PubMed] [Google Scholar]
- Cao D, Fukuchi KI, Wan H, Kim H, Li L. Lack of LDL receptor aggravates learning deficits and amyloid deposits in Alzheimer transgenic mice. Neurobiol Aging. 2006;27:1632–1643. doi: 10.1016/j.neurobiolaging.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, Reiner PB. Beta-amyloid efflux mediated by p-glycoprotein. J Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- Deane R, Du YS, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Lue LF, Yan SD, Stern DM, Walker DG. Preventing activation of receptor for advanced glycation end products in Alzheimer’s disease. Curr Drug Targets CNS Neurol Disord. 2005;4:249–266. doi: 10.2174/1568007054038210. [DOI] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, Shen W, Dunlop NM, Gao JL, Murphy PM, Oppenheim JJ, Wang JM. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J Neurosci. 2001;21:RC123–127. doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Yuan M, Zhang Z, Paganetti PA, Sturchler-Pierrat C, Staufenbiel M, Mautino J, Vigo FS, Sommer B, Yankner BA. Amyloid beta interacts with the amyloid precursor protein: a potential toxic mechanism in Alzheimer’s disease. Nat Neurosci. 2000;3:460–464. doi: 10.1038/74833. [DOI] [PubMed] [Google Scholar]
- White AR, Maher F, Brazier MW, Jobling MF, Thyer J, Stewart LR, Thompson A, Gibson R, Masters CL, Multhaup G, Beyreuther K, Barrow CJ, Collins SJ, Cappai R. Diverse fibrillar peptides directly bind the Alzheimer’s amyloid precursor protein and amyloid precursor-like protein 2 resulting in cellular accumulation. Brain Res. 2003;966:231–244. doi: 10.1016/s0006-8993(02)04173-2. [DOI] [PubMed] [Google Scholar]
- Weller RO, Massey A, Kuo YM, Roher AE. Cerebral amyloid angiopathy: accumulation of A beta in interstitial fluid drainage pathways in Alzheimer’s disease. Ann NY Acad Sci. 2000;903:110–117. doi: 10.1111/j.1749-6632.2000.tb06356.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Otte-Holler I, Wesseling P, de Waal RM, Boelens WC, Verbeek MM. Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer’s disease brains. Neuropathol Appl Neurobiol. 2006;32:119–130. doi: 10.1111/j.1365-2990.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM. Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res. 2006;1089:67–78. doi: 10.1016/j.brainres.2006.03.058. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, Kusters B, Maat-Schieman ML, de Waal RM, Verbeek MM. Small heat shock protein HspB8: its distribution in Alzheimer’s disease brains and its inhibition of amyloid-beta protein aggregation and cerebrovascular amyloid-beta toxicity. Acta Neuropathol (Berl) 2006;111:139–149. doi: 10.1007/s00401-005-0030-z. [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Wagner SL, Suzuki M, Choi BH, Farrow JS, Geddes JW, Cotman CW, Cunningham DD. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature. 1989;341:546–549. doi: 10.1038/341546a0. [DOI] [PubMed] [Google Scholar]
- Verbeek MM, Otte-Holler I, Wesseling P, Ruiter DJ, de Waal RM. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta 1. Am J Pathol. 1994;144:372–382. [PMC free article] [PubMed] [Google Scholar]
- Verbeek MM, Otte-Holler I, Fransen JA, de Waal RM. Accumulation of the amyloid-beta precursor protein in multivesicular body-like organelles. J Histochem Cytochem. 2002;50:681–690. doi: 10.1177/002215540205000509. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, Janssen I, Hoozemans JJ, De Groot CJ, Hack CE, Eikelenboom P. Complement C1-inhibitor expression in Alzheimer’s disease. Acta Neuropathol (Berl) 1998;96:287–296. doi: 10.1007/s004010050896. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Otte-Holler I, Davis J, Van Nostrand WE, de Waal RM, Verbeek MM. Apolipoprotein E genotype regulates amyloid-beta cytotoxicity. J Neurosci. 2005;25:3621–3627. doi: 10.1523/JNEUROSCI.4213-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorina EM, Sovershaev MA, Bjorkoy G, Gruber FX, Olsen JO, Parhami-Seren B, Mann KG, Osterud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25:1493–1498. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorina EM, Sovershaev MA, Osterud B. In-cell Western assay: a new approach to visualize tissue factor in human monocytes. J Thromb Haemost. 2006;4:614–620. doi: 10.1111/j.1538-7836.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- Bu G. The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int Rev Cytol. 2001;209:79–116. doi: 10.1016/s0074-7696(01)09011-8. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, Van Eldik LJ. Apolipoprotein E and apolipoprotein E receptors modulate A beta-induced glial neuroinflammatory responses. Neurochem Int. 2001;39:427–434. doi: 10.1016/s0197-0186(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Yazawa H, Yu ZX, Takeda, Le Y, Gong W, Ferrans VJ, Oppenheim JJ, Li CC, Wang JM. Beta amyloid peptide (Abeta42) is internalized via the G-protein-coupled receptor FPRL1 and forms fibrillar aggregates in macrophages. FASEB J. 2001;15:2454–2462. doi: 10.1096/fj.01-0251com. [DOI] [PubMed] [Google Scholar]
- Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology. 1997;49:56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer’s disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem. 2003;278:2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du YS, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Ishiguro K, Sugihara S, Nakazato Y, Kawarabayashi T, Sun X, Hirai S. Presence of apolipoprotein E on extracellular neurofibrillary tangles and on meningeal blood vessels precedes the Alzheimer beta-amyloid deposition. Acta Neuropathol (Berl) 1994;88:413–419. doi: 10.1007/BF00389492. [DOI] [PubMed] [Google Scholar]
- Urmoneit B, Prikulis I, Wihl G, D’Urso D, Frank R, Heeren J, Beisiegel U, Prior R. Cerebrovascular smooth muscle cells internalize Alzheimer amyloid beta protein via a lipoprotein pathway: implications for cerebral amyloid angiopathy. Lab Invest. 1997;77:157–166. [PubMed] [Google Scholar]
- Bu G, Cam J, Zerbinatti C. LRP in amyloid-beta production and metabolism. Ann NY Acad Sci. 2006;1086:35–53. doi: 10.1196/annals.1377.005. [DOI] [PubMed] [Google Scholar]
- Rensink AA, Otte-Holler I, de Boer R, Bosch RR, ten Donkelaar HJ, de Waal RM, Verbeek MM, Kremer B. Insulin inhibits amyloid beta-induced cell death in cultured human brain pericytes. Neurobiol Aging. 2004;25:93–103. doi: 10.1016/s0197-4580(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Jung SS, Van Nostrand WE. Humanin rescues human cerebrovascular smooth muscle cells from Abeta-induced toxicity. J Neurochem. 2003;84:266–272. doi: 10.1046/j.1471-4159.2003.01524.x. [DOI] [PubMed] [Google Scholar]
- van Duinen SG, Castano EM, Prelli F, Bots GT, Luyendijk W, Frangione B. Hereditary cerebral hemorrhage with amyloidosis in patients of Dutch origin is related to Alzheimer disease. Proc Natl Acad Sci USA. 1987;84:5991–5994. doi: 10.1073/pnas.84.16.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.