Abstract
The hyaluronan receptor CD44 plays an important role in facilitating invasion and metastasis of a variety of tumors, including breast carcinomas. CD44 functions as a bioactive signaling transmitter. Although a number of studies have implicated CD44 in breast tumor invasion, the evidence is still circumstantial. We have developed a tetracycline-regulated CD44s (standard form) system in the weakly metastatic breast cancer cell MCF7, which exhibits low endogenous expression of CD44 and generated a new cell line, MCF7F-B5. Induction of CD44s alone affected the growth characteristics of MCF7F-B5 cells by increasing their abilities to proliferate, migrate, and invade in vitro. In addition, we have identified and validated cortactin as a novel transcriptional target of hyaluronan/CD44s signaling in underpinning breast tumor invasion. To test these observations in vivo, we developed a doxycycline (DOX)-regulated CD44s breast cancer xenograft model. Induction of CD44s did not affect the growth rate or local invasion of the primary tumor. However, although no mice from the +DOX group developed metastasis, 8 of 11 mice from the −DOX group developed secondary tumors to the liver only. Interestingly, metastatic breast tumors expressed high levels of CD44. This study provides in vivo evidence for the role of the standard form of CD44 in promoting breast tumor invasion and metastasis to the liver.
Breast cancer cells metastasize from the original tumor site through the vasculature to distant organs such as the liver, lungs, brain, and bones. These cells also invade and exfoliate into body cavities, especially the pleural space, where they grow in suspension within effusions.1 Invasion is the defining event for cancer and the recurring step in the metastatic process. Cell invasion depends, in great part, on the ability of invading cells to attach to and then detach from various types of cells and extracellular components. Although moving to secondary sites, tumor cells use a new set of adhesion, migration, and homing proteins. The adhesion molecule CD44 (also known as homing protein, PGP-1, Hermes antigen, and HUTCH-1) is the principal cell surface receptor for hyaluronic acid (HA), although other extracellular matrix (ECM) proteins, including collagen, fibronectin, and osteopontin, can bind CD44.2 Via its interaction with HA, CD44 functions as a bioactive signaling transmitter, leading to diverse effects on cellular adhesion, migration, and invasion—all of which are important processes in cancer progression as well as in hematopoietic development and wound healing.3,4,5
CD44 is a ubiquitous multistructural and multifunctional cell surface adhesion molecule involved in cell-cell and cell-matrix interactions. It is the most studied alternatively spliced gene in cancer. Of its 20 exons, the first and last five exons are constant, whereas the 10 intermediate exons are subject to alternative splicing, resulting in the generation of a variable region. Differential utilization of the 10 variable region exons generates multiple isoforms of different molecular sizes (85 to 230 kDa), as do variations in N-glycosylation, O-glycosylation, and glycosaminoglycanation. This fact implicates CD44 in a variety of responses, including lymphocyte homing, hematopoiesis, inflammation, tumorigenesis, angiogenesis, and metastasis. The smallest CD44 molecule (85 to 95 kDa), which lacks the entire variable region, is the standard form (CD44s). CD44s is a single-chain molecule composed of a distal extracellular domain (containing the ligand-binding sites), a membrane-proximal region, a transmembrane-spanning domain, and a cytoplasmic tail. A CD44 isoform containing the last three exon products of the variable region, CD44V8–10 (also known as epithelial CD44 or CD44E), is preferentially expressed on epithelial cells. Whereas the CD44s is widely expressed and HA is ubiquitous in most extracellular spaces, variant isoforms of CD44 have restricted expression to specific conditions, such as transformation, wound healing, and lymphocyte activation.6
In theory, CD44 has the basic structure necessary to function in signal transduction. However, the CD44 cytoplasmic tail exhibits no inherent receptor kinase or phosphatase activity. ECM outside-in CD44 signaling is communicated through various mechanisms: 1) direct CD44 signaling, for which HA binding initiates extracellular clustering of CD44, resulting in the activation of kinases, such as c-Src and FAK as well as Rho and Rac7,8,9; this activation leads to enhanced association of CD44 into actin cytoskeleton complexes as well as recruitment and activation of additional signaling partners that potentiate cell migration10; 2) CD44 serving as a co-receptor physically linked to other classical signaling receptors, such as c-Met,11 members of the ErbB family of receptor tyrosine kinases,12,13 and transforming growth factor-βR1,14,15,16 and in the process, facilitating the association of intracellular mediators of signal transduction; 3) CD44 functioning as a docking protein for pericellular proteins, such as matrix metalloproteinases,17,18 or for cytoplasmic proteins, such as kinases, Smad1, actin-binding proteins, and other adapter proteins that can subsequently activate signaling pathways; 4) direct CD44-mediated signaling occurring as a two-step process: γ-secretase cleavage of the transmembrane domain and the subsequent release of CD44 intracellular domain, which exhibits nuclear translocation and functions as a transcription factor of target genes, such as CD44 itself.19
Although a number of studies have implicated CD44s as a facilitator of breast tumor invasion,5,20,21 the evidence is circumstantial22 and based on the clinical correlation between metastatic capacity and the expression of CD44s and specific splice variants.23 Nevertheless, strong evidence from our own work and others has suggested that CD44s-mediated adhesion and signaling are required for cell growth and the dissemination of breast tumors.23,24,25 Therefore, we focused our investigation on CD44s. Having recently developed a tetracycline (Tet)-Off-regulated expression of CD44s in the poorly invasive breast cancer cell line MCF-7, we were able to show that Tet-regulated induction of CD44 expression increased migration and invasion of the inducible cells. These observations suggested that CD44s can promote breast tumor cell invasion in vitro.26 To understand better the function of the standard form, CD44s, in breast cancer invasion and metastasis, and to elucidate further the downstream signaling of this adhesion molecule, we developed a Tet-regulated induction of CD44s system in vivo, using a breast tumor cell xenograft model.
Materials and Methods
Cells and Compounds
The breast cancer cell line MCF7F-B5 clone with inducible CD44s expression26 was maintained in Dulbecco’s modified Eagle’s medium-supplemented media containing 10% (v/v) fetal calf serum, 2.5 μg/ml Tet, 100 μg/ml G418 [Roche Diagnostics Ltd. (GmBH), Lewes, UK], and 1 μg/ml puromycin. Chemicals were supplied by Sigma Chemical Co. (St. Louis, MO) unless otherwise stated. HA of molecular weight 220 kDa and medical grade purity was purchased from Lifecore Biomedical Inc. (Chaska, MN).
Animal Studies
The nonmetastatic MCF-7 continuous line of human breast cancer cells requires that immune-deficient mice receive supplemental estrogen so that inocula can produce progressively growing tumors. Therefore, 24 female SCID (CB 17 immune-deficient) mice purchased from Harlan (Oxon, UK) were randomly divided into two groups (n = 12 per group). One week after their arrival to the animal facility, they were implanted with a 60-day, slow release of 17-β-estradiol at 1.7 mg/pellet into a small subcutaneous pocket fashioned between the shoulder blades (behind the neck). For in vivo induction of the Tet-responsive promoter, the control group received doxycycline (+DOX) daily by gavages (2 mg/ml in 2.5% sucrose), whereas the other group received 2.5% sucrose only (−DOX). Three days after estrogen-pellet implantation, 106 of MCF7F-5B cells in 0.2 ml of serum-free culture medium were injected subcutaneously. After tumors became visible, body weight and tumor size were measured twice a week. Five weeks later, all animals developed primary tumors at the site of inoculation. The mice were humanely sacrificed, and the primary tumors were removed and dissected into two pieces, one for extraction of protein lysates and the other to be fixed in formalin for histology. Brain, bone, lung, and liver tissues (potential target tissues for breast tumor metastasis) were also removed and fixed in formalin for analysis of breast metastasis.
Histology and Immunohistochemistry (IHC)
IHC assays were performed as we have previously described27 with slight modifications. Briefly, adjacent sections were examined for CD44 expression using mouse anti-human CD44 antibody (1:30 dilution, clone DF1485; DAKO, Carpinteria, CA). Staining was performed using the Ventana Nexus immunostainer and Ventana detection kit system (Ventana Medical Systems Inc., Tucson, AZ). Adjacent sections were also stained with hematoxylin and eosin for histology.
Western Blotting
The MCF7F-B5 cells were induced after withdrawal of Tet at different time points, and protein lysates were collected as previously described.26 A primary tumor sample from each mouse was homogenized, and proteins were extracted with a lysis buffer and precipitated using RIPA buffer. Protein samples were centrifuged to remove cell debris, and the final concentration in the resulting supernatant was determined using the BCA protein assay reagent (Pierce, Rockford, IL). Protein samples (30 μg) were boiled for 5 minutes in an equal volume of reducing buffer (5 mmol/L Tris/HCl pH 7.4, 4% (w/v) sodium dodecyl sulfate, 20% (v/v) glycerol, 10% (v/v) mercaptoethanol, 0.2% (w/v) bromophenol blue), resolved on 12% polyacrylamide gels, and electroblotted onto nitrocellulose membranes. Membranes were probed with mouse anti-human CD44 monoclonal antibody (1:500 dilution; R&D Systems, Minneapolis, MN) and then treated with a sheep anti-mouse IgG/horseradish peroxidase conjugate (1:2000 dilution; Amersham, Buckinghamshire, UK); immunoreactivity was detected using chemiluminescence (Super signal, Pierce). Equal loading of the protein samples was assessed by reprobing the membrane with a 1:2000 dilution of the β-tubulin antibody (Sigma).
Results
In Vitro Characterization of the Tet Off-Regulated CD44s Expression System
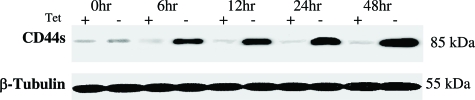
We have previously generated a Tet Off-regulated CD44 expression in the parental MCF-7 cells (with minimal CD44 expression) and obtained the inducible MCF7F-B5 breast cancer cell line.26 To ensure that this system is functional in vitro, the expression of CD44 protein in the MCF7F-B5 clone was studied throughout a 48-hour time course in the presence and absence of Tet. Protein was extracted at 0, 6, 12, 18, 24, and 48 hours (Figure 1), resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and probed for CD44 expression using a mouse anti-human CD44 monoclonal antibody. In the absence of Tet, a single immunoreactive band was detected at a molecular mass equivalent to 85 kDa, consistent with the presence of CD44s. The level of expression of CD44s showed a time-dependent increase with peak overexpression that was detectable at 48 hours and was maintained out to 96 hours in this system (data not shown). However, MCF7F-B5 cells cultured in Tet showed barely detectable CD44 expression (Figure 1), indicating that the Tet-regulated CD44s suppression system was functional in the MCF7-B5 breast cancer cell line.
Figure 1.
Induction of CD44s expression in the Tet-regulated MCF7F-B5 breast cancer cell line. The immunoblot demonstrates a time-dependent induction of CD44s (85-kDa band) protein expression in MCF7F-B5 cells after the removal of Tet (−) from the growth media, after the indicated time in hours.
In Vivo Validation of the Tet Off-Regulated CD44s Expression System
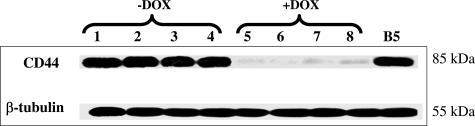
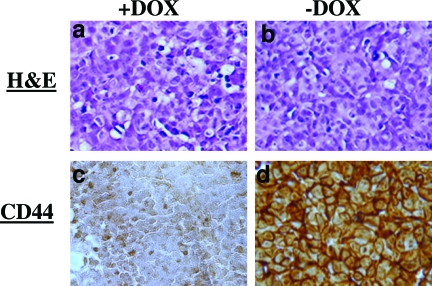
To test whether the in vitro Tet Off-regulated CD44 expression system is also functional in vivo, we developed the Tet Off-regulated CD44 expression system, using a xenograft breast cancer model by subcutaneous injection of the MCF7F-B5 cells into immune-deficient mice fed with or without DOX. Five weeks after the inoculation of MCF7–5B clone, the primary tumors were collected from the CD44-induced group (−DOX) as well as the CD44-noninduced group (+DOX) and were analyzed for CD44s expression by Western blot as well as IHC. Protein was extracted from the primary tumors, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and probed for CD44 expression, using a mouse anti-human CD44 mAb. Compared to CD44s expression in the MCF7-B5 cell cultured in the absence of Tet (positive control), Figure 2 shows a barely detectable expression of CD44 in the +DOX group. In contrast, strong expression of CD44 was found in the primary tumors from the −DOX group. Similar results were obtained by IHC (Figure 3), and CD44-immunostaining detected on the cell membrane was highly expressed in the primary breast tumor tissue from the −DOX group. Together, these data indicate that the inducible CD44s-expression breast model is properly functional in vivo.
Figure 2.
In vivo characterization of the inducible Tet Off-regulated CD44 expression system by Western blot analysis using MCF7-B5 breast cancer xenograft model. Compared to the expression of CD44s induced in the MCF7F-B5 (B5) cell line, CD44 was highly induced in the primary tumors from the mice not supplemented with DOX (−DOX), but very low expression was detected in the primary tumors of mice that were given DOX (+DOX). Lanes 1 to 4: four representative mice from −DOX group; lanes 5 to 8: four representative mice from +DOX group.
Figure 3.
In vivo validation of the inducible Tet Off-regulated CD44 expression system by IHC using MCF7F-B5 breast cancer xenograft model. a and b: Primary tumors from the CD44 on-induced group (+DOX) and the CD44-induced group (−DOX), respectively, showing H&E staining. c and d: The same primary tumors showing IHC analysis. Compared to +DOX group, CD44 was highly expressed in the primary tumors from the CD44-induced group (−DOX). Original magnifications, ×200.
Effect of CD44 Induction on Metastasis of MCF7F-B5 Breast Cancer Cells
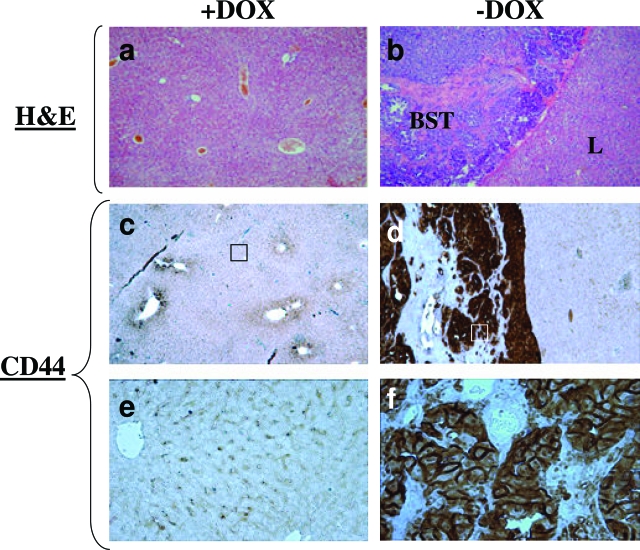
Using in vitro cell migration and invasion assays, we previously studied the effect of induced CD44s expression on the invasive potential of MCF7F-B5 cells, and this study showed that induction of CD44 expression in the poorly invasive MCF7F-B5 cells increased the migratory and invasive potential of these cells.26 To test this observation in vivo, we examined mice for breast cancer metastases to different tissues after primary tumors had been established after the subcutaneous injection of the MCF7F-B5 cells into the immune-deficient mice fed with or without DOX. Induction of CD44s did not affect the growth rate or local invasion of the primary tumor (data not shown). Histopathological analysis of the collected organs (brain, bone, liver, and lung) from the −DOX group showed no micrometastases. Surprisingly, whereas none of the +DOX mice showed any metastasis (Table 1; Figure 4), 8 of 11 mice from the −DOX group had a large secondary tumor to the liver. When secondary breast tumors to the liver were analyzed by IHC for CD44s expression, CD44s immunostaining was detected in the cells of breast secondary tumor tissue, but no CD44s expression was found in the adjacent liver tissue (Figure 4). Collectively, these data suggest that up-regulation of the CD44s in the MCF7F-B5 cells promotes breast tumor metastasis.
Table 1.
Metastasis of the Primary Breast Tumors to the Liver after Induction of CD44s
| Tumor type | Primary tumor | Metastasis to the liver |
|---|---|---|
| Group | ||
| +DOX | 12 of 12 | 0 of 12 |
| −DOX | 11 of 11 | 8 of 11 |
Upon induction of CD44s (−DOX group), the primary breast tumors generated from subcutaneous injection of the MCF7F-B5 cells metastasized to the liver only in 8 of 11 mice, whereas no mice from the control group (+DOX) developed any metastasis.
Figure 4.
In vivo induction of CD44s promotes metastasis of breast cancer cell line MCF7F-B5 to the liver. The breast secondary tumor (BST) to the liver from −DOX group (CD44s-induced) showed intense CD44 expression (d and f) compared to the liver tissue from the +DOX control group (CD44s-inhibited) where CD44 was absent (c and e). e and f represent magnified portion of c and d, respectively. Original magnifications, ×200 (e, f).
Discussion
Mammary cancer is the most common cancer among women, excluding nonmelanoma skin cancers. Although 50% of mammary cancers are surgically curable, 50% of patients experience metastasis within 5 years after surgery. Key to increasing the patient’s quality of life and long-term survival is the development of improved early detection methods and targeted therapies for controlling cancer metastasis. As a crucial first step, we are attempting to gain a better understanding of the molecular mechanisms associated with breast cancer invasion/metastasis. Regulation of cell-cell and cell-matrix interactions via cell adhesion molecules is a critical step during the metastatic spread of tumors. CD44 plays an essential role in regulating invasion and metastasis of breast tumors.5,28,29 Although upstream signaling mechanisms controlling CD44 activity have been well established,7 the downstream signaling processes are poorly understood.
Recently, we have generated a Tet Off-regulated CD44 expression in a weakly metastatic breast cancer cell line exhibiting low endogenous expression of CD44 and have demonstrated that induction of CD44s increased the migration and invasiveness of MCF7F-B5 cells through an experimental matrix.26 To test this in vitro observation and determine the precise role of CD44s in tumor growth and metastasis, we developed an in vivo Tet Off-regulated CD44 expression system, using a breast cancer xenograft mouse model. Comparing these mice to control mice, we showed that: induction of CD44s resulted in metastasis of MCF7F-B5-primary breast tumors to the liver but did not affect either the formation or growth rate of the primary tumor, and both primary and secondary tumors expressed high levels of CD44. These data confirmed our previous in vitro results,26 clearly demonstrating that up-regulation of CD44s can promote breast tumor metastasis to the liver. To understand better the molecular mechanisms implicated in CD44s downstream signaling-mediated breast tumor invasion, we used gene profiling technology, which allowed us to identify a number of potential CD44s downstream transcriptional targets,26 and experiments aiming to validate their functional role in CD44s-promoted breast cell invasion and metastasis are underway.
Several researchers have concluded that CD44s promotes tumor progression and metastasis, whereas others maintain that CD44s inhibits this process. Using MMTV-PyV mT mice crossed onto a CD44−/− background, Lopez and colleagues30 reported the absence of CD44-induced breast cancer metastasis to the lung, suggesting that HA binding to an alternate receptor, such as RHAMM or LYVE-1, may attenuate breast cancer cell invasion. It has to be emphasized that, although breast cancer cell lines can express both standard and variant isoforms, the CD44−/− mouse model cells are lacking all forms of CD44.30 Compared to the MMTV-PyV mT/CD44−/− mouse model,30 the advantage of the Tet Off-regulated CD44 expression breast cancer xenograft mouse model described in the present study is that the effect of a selected human CD44, CD44s, can be evaluated. Several studies have provided strong evidence to support our findings that CD44s can facilitate breast tumor cell invasion and metastasis.30,31,32,33,34 The hypothesis that CD44 plays a potential role in metastasis originated from the simple observation that the CD44v6 variant conferred metastatic ability to a previously nonmetastatic rat pancreatic carcinoma cell line.31 Conversely, antisense CD44 transfection has been shown to inhibit tumor growth and metastasis in highly metastatic mammary carcinoma cells.35 Human breast carcinoma samples examined by different techniques were found to contain primarily CD44E isoform in the normal breast tissue whereas metastatic breast carcinomas appeared to overexpress CD44s and variant isoforms.5
Previous studies using IHC have reported a wide range of anti-CD44s positivity in invasive breast carcinoma, from 26 to 62%.36,37,38,39 The expression of CD44s and CD44v6 was associated with the invasion and metastasis of breast carcinoma.40 CD44-HA interaction seemed to be cell-type-specific and dependent on CD44 isoform expression during cancer progression and metastatic spread. Also, HA has been shown to promote breast cancer cell invasion in vitro through activation of CD44.41 Additional studies have also identified a number of partners that CD44 may interact with to promote cell signaling that mediates tumor progression. Examples include c-Src, ErbB2, ankyrin, and the ERM proteins.7,12,42 Whereas HA is highly expressed and even induced in the ECM of these tumors, induction of its primary receptor in weakly invasive MCF7F-B5 breast cancer cell line resulted in an increase in tumor metastasis to the liver. This finding implicates CD44s/HA interaction in breast tumor invasion. Previous studies have established a positive correlation between cell invasiveness and increased expression of HAS2 (HA synthase 2), HA, HYAL2 (hyaluronidase 2), and CD44.43 Udabage and colleagues43 have recently shown that antisense inhibition of HAS2 resulted in accumulation of high molecular weight HA and a decrease in CD44 and HYAL2, resulting in attenuated cellular proliferation and in vitro invasiveness, and inhibited the formation of primary and secondary tumors in vivo.44 Expression levels of HAS2, HA, and HYAL2 will be examined in the same samples obtained in the present study. These data support our finding that CD44s might play an important role, not only in the initiation of secondary tumors but also in their growth. Furthermore, our previous in vitro model strongly supports the present data.26 Induction of CD44 expression on the weakly motile MCF7F-B5 cells increased the migratory and invasive properties of this breast cancer cell line in HA-supplemented Matrigel. In addition, depletion of CD44 expression on the breast cancer cell lines MDA-MB-231 and MDA-MB-157 cells both reduced the invasiveness of these cells and decreased the adhesion of these cells to human bone marrow endothelial cells.45 Conversely, the adhesion of each of the CD44-negative breast cancer and prostate cancer cell lines (T47D, MCF-7, and DU145) to human bone marrow endothelial cells was dramatically increased after transfection of these cells to express CD44s.45
Studies using MCF-7 breast xenograft models have observed metastasis to the liver and other organs such as bone and lung.46,47 In the present study, metastasis of the primary breast tumor cells MCF7F-B5 was seen in the liver only. This observation might be explained by the activation of specific pathway(s) on induction of CD44s in the MCF7-B5 cells leading to a liver-specific metastasis. Breast cancer cells with CD44+/CD24− subpopulation have been shown to express higher levels of proinvasive genes and have highly invasive properties,21 supporting cortactin as a newly identified target for CD44-promoted breast tumor invasion/metastasis.26 Ongoing validation experiments in our laboratory are aiming to further identify potential CD44s downstream target genes that might play a key role in the organotropism of breast cancer metastasis.48 Although metastasis to the liver is observed infrequently in patients with breast cancer at initial presentation, it portends a worse clinical outcome.49 Metastatic breast cancer to the liver contributes to a shorter median survival rate, reported to be in the range of 3 to 14 months.50 Although the liver is the site of metastasis in 5 to 20% of women with metastatic breast cancer,50 liver metastasis rarely occurs in women with ductal carcinoma in situ. Our data indicate that CD44s might be involved in facilitating aggressive dissemination of breast cancer tumors to the liver.
In summary, our model provides compelling evidence for a role of CD44s in promoting breast cancer metastasis to the liver. The cell adhesion mechanisms play an important role in tumor invasion, including those mediating the attachment of tumor cells to the ECM and basement membranes. Both the degradation of basement membranes and migration through the ECM are facilitated by the interaction of CD44 with hyaluronic acid. Several lines of evidence from our work and others suggest that up-regulation of CD44s in the primary tumors provides the cells with an increased potential of adhesion, migration, and invasion. However, more basic studies are needed to understand better the role of specific CD44 isoforms, induced selectively or in combination, in cell invasion signaling. In addition, validation of these mechanisms in human clinical tumor specimens is required to shed light on the precise role and signaling mechanism of CD44 isoforms in breast tumor progression and metastasis.
Acknowledgments
We thank Dr. Alain Sarasin for his critical review of the manuscript and Dr. D.J. Waugh for supplying the CD44-B5 clone.
Footnotes
Address reprint requests to Allal Ouhtit, M.Ph., Ph.D., Stanley S. Scott Cancer Center, Louisiana State University Health Science Center CSRB Building, Room no. 748C, 533 Bolivar St., New Orleans, LA 70112. E-mail: aouhti@lsuhsc.edu.
Supported by the Louisiana Cancer Research Consortium in New Orleans (to A.O.) and the Egyptian Ministry of Higher Education (fellowship to M.A.).
References
- Shulman LN, Sugarbaker DJ. Malignant effusions. Harris JR, Lippman ME, Morrow M, Hellman S, editors. Philadelphia: Lippincott-Raven,; Diseases of the Breast. 1996:pp 833–840. [Google Scholar]
- Wai PY, Kuo PC. The role of osteopontin in tumor metastasis. J Surg Res. 2004;121:228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- Iida N, Bourguignon LY. New CD44 splice variants associated with human breast cancers. J Cell Physiol. 1995;162:127–133. doi: 10.1002/jcp.1041620115. [DOI] [PubMed] [Google Scholar]
- Naor D, Sionov RV, Ish-Shalom D. CD44, structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- Ponta H, Wainwright D, Herrlich P. The CD44 protein family. Int J Biochem Cell Biol. 1998;30:299–305. doi: 10.1016/s1357-2725(97)00152-0. [DOI] [PubMed] [Google Scholar]
- Bourguignon LYW, Zhu H, Shao L, Chen Y. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem. 2001;276:7327–7336. doi: 10.1074/jbc.M006498200. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Kitagawa M, Nakamura S, Azuma K, Ishii G, Higashi M, Kishi H, Hiwasa T, Koda K, Nakajima N, Harigaya K. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Lett. 2002;528:101–108. doi: 10.1016/s0014-5793(02)03262-3. [DOI] [PubMed] [Google Scholar]
- Murai T, Miyazaki Y, Nishinakamura H, Sugahara KN, Miyauchi T, Sako Y, Yanagida T, Miyasaka M. Engagement of CD44 promotes Rac activation and CD44 cleavage during tumor cell migration. J Biol Chem. 2004;279:4541–4550. doi: 10.1074/jbc.M307356200. [DOI] [PubMed] [Google Scholar]
- Sohara Y, Ishiguro N, Machida K, Kurata H, Thant AA, Senga T, Matsuda S, Kimata K, Iwata H, Hamaguchi M. Hyaluronan activates cell motility of v-Src-transformed cells via Ras-mitogen-activated protein kinase and phosphoinositide 3-kinase-Akt in a tumor-specific manner. Mol Biol Cell. 2001;12:1859–1868. doi: 10.1091/mbc.12.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem. 1997;272:27913–27918. doi: 10.1074/jbc.272.44.27913. [DOI] [PubMed] [Google Scholar]
- Yu WH, Woessner JF, Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 2002;16:307–323. doi: 10.1101/gad.925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor-β receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- Ito T, Williams JD, Fraser D, Phillips AO. Hyaluronan attenuates transforming growth factor-β1-mediated signaling in renal proximal tubular epithelial cells. Am J Pathol. 2004;164:1979–1988. doi: 10.1016/s0002-9440(10)63758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Williams JD, Fraser DJ, Phillips AO. Hyaluronan regulates transforming growth factor-β1 receptor compartmentalization. J Biol Chem. 2004;279:25326–25332. doi: 10.1074/jbc.M403135200. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, Tojo H, Yana I, Seiki M. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 2002;21:3949–3959. doi: 10.1093/emboj/cdf411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami D, Okamoto I, Nagano O, Kawano Y, Tomita T, Iwatsubo T, De Strooper B, Yumoto E, Saya H. Presenilin-dependent g-secretase activity mediates the intramembranous cleavage of CD44. Oncogene. 2003;22:1511–1516. doi: 10.1038/sj.onc.1206298. [DOI] [PubMed] [Google Scholar]
- Khan SA, Cook AC, Kappil M, Gunthert U, Chambers AF, Tuck AB, Denhardt DT. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: novel post-transcriptional, post-translational regulation. Clin Exp Metastasis. 2005;22:663–673. doi: 10.1007/s10585-006-9007-0. [DOI] [PubMed] [Google Scholar]
- Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R, Jr, Badve S, Nakshatri H. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götte M, Yip GW. Heparanase, hyaluronan, and CD44 in cancer: a breast carcinoma perspective. Cancer Res. 2006;66:10233–10237. doi: 10.1158/0008-5472.CAN-06-1464. [DOI] [PubMed] [Google Scholar]
- Bànkfalvi A, Terpe HJ, Breukelmann D, Bier B, Rempe D, Pschadka G, Krech R, Bocker W. Gains and losses of CD44 expression during breast carcinogenesis and tumor progression. Histopathology. 1998;33:107–116. doi: 10.1046/j.1365-2559.1998.00472.x. [DOI] [PubMed] [Google Scholar]
- Sy MS, Guo YJ, Stamenkovic I. Inhibition of tumor growth in vivo with a soluble CD44-immunoglobulin fusion protein. J Exp Med. 1992;176:623–627. doi: 10.1084/jem.176.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RM, Yu Q, Stamenkovic I, Toole BP. Perturbation of hyaluronan interactions by soluble CD44 inhibits growth of murine mammary carcinoma cells in ascites. Am J Pathol. 2000;156:2159–2167. doi: 10.1016/S0002-9440(10)65086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, McFarlane S, Mulligan K, Gillespie H, Draffin J, Trimble A, Ouhtit A, Johnston PG, Harkin DP, McCormick D, Waugh DJ. Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene. 2006;25:6079–6091. doi: 10.1038/sj.onc.1209628. [DOI] [PubMed] [Google Scholar]
- Ouhtit A, Muller HK, Davis DW, Ullrich SE, McConkey D, Ananthaswamy HN. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol. 2000;156:201–207. doi: 10.1016/S0002-9440(10)64720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Oliferenko S, Kaverina I, Small JV, Huber LA. Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J Cell Biol. 2000;148:1159–1164. doi: 10.1083/jcb.148.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–6763. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- Choi SH, Takahashi K, Eto H, Yoon SS, Tanabe KK. CD44s expression in human colon carcinomas influences growth of liver metastases. Int J Cancer. 2000;85:523–526. doi: 10.1002/(sici)1097-0215(20000215)85:4<523::aid-ijc13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Günthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Gunja-Smith Z, Iida N, Zhu HB, Young LJ, Muller WJ, Cardiff RD. CD44v(3,8-10) is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J Cell Physiol. 1998;176:206–215. doi: 10.1002/(SICI)1097-4652(199807)176:1<206::AID-JCP22>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Iida N, Bourguignon LY. Coexpression of CD44 variant (v10/ex14) and CD44S in human mammary epithelial cells promotes tumorigenesis. J Cell Physiol. 1997;171:152–160. doi: 10.1002/(SICI)1097-4652(199705)171:2<152::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Yu Q, Toole BP, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–1996. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiter S, Arch R, Reber S, Komitowski D, Hofmann M, Ponta H, Herrlich P, Matzku S, Zoller M. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177:443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bànkfalvi A, Terpe HJ, Breukelmann D, Bier B, Rempe D, Pschadka G, Krech R, Bocker W. Gains and losses of CD44 expression during breast carcinogenesis and tumour progression. Histopathology. 1998;33:107–116. doi: 10.1046/j.1365-2559.1998.00472.x. [DOI] [PubMed] [Google Scholar]
- Berner HS, Suo Z, Risberg B, Villman K, Karlsson MG, Nesland JM. Clinicopathological associations of CD44 mRNA and protein expression in primary breast carcinomas. Histopathology. 2003;42:546–554. doi: 10.1046/j.1365-2559.2003.01622.x. [DOI] [PubMed] [Google Scholar]
- Friedrichs K, Franke F, Lisboa BW, Kugler G, Gille I, Terpe HJ, Holzel F, Maass H, Gunthert U. CD44 isoforms correlate with cellular differentiation but not with prognosis in human breast cancer. Cancer Res. 1995;55:5424–5433. [PubMed] [Google Scholar]
- Lyzak JS, Yaremko ML, Recant W, Baunoch DA, Joseph L. Role of CD44 in nonpalpable T1a and T1b breast cancer. Hum Pathol. 1997;28:772–778. doi: 10.1016/s0046-8177(97)90148-9. [DOI] [PubMed] [Google Scholar]
- Herrera-Gayol A, Jothy S. Effects of hyaluronan on the invasive properties of human breast cancer cells in vitro. Int J Exp Pathol. 2001;82:193–200. doi: 10.1046/j.1365-2613.2001.iep0082-0193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udabage L, Brownlee GR, Nilsson SK, Brown TJ. The over-expression of HAS2. Hyal-2 and CD44 is implicated in the invasiveness of breast cancer. Exp Cell Res. 2005;310:205–217. doi: 10.1016/j.yexcr.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Li Y, Li L, Brown TJ, Heldin P. Silencing of hyaluronan synthase 2 suppresses the malignant phenotype of invasive breast cancer cells. Int J Cancer. 2007;120:2557–2567. doi: 10.1002/ijc.22550. [DOI] [PubMed] [Google Scholar]
- Draffin JE, McFarlane S, Hill A, Johnston PG, Waugh DJ. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. 2004;64:5702–5711. doi: 10.1158/0008-5472.CAN-04-0389. [DOI] [PubMed] [Google Scholar]
- Shafie SM, Liotta LA. Formation of metastasis by human breast carcinoma cells (MCF-7) in nude mice. Cancer Lett. 1980;11:81–87. doi: 10.1016/0304-3835(80)90097-x. [DOI] [PubMed] [Google Scholar]
- Kurebayashi J, McLeskey SW, Johnson MD, Lippman ME, Dickson RB, Kern FG. Quantitative demonstration of spontaneous metastasis by MCF-7 human breast cancer cells cotransfected with fibroblast growth factor 4 and LacZ. Cancer Res. 1993;53:2178–2187. [PubMed] [Google Scholar]
- Lu X, Kang Y. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2007;12:153–162. doi: 10.1007/s10911-007-9047-3. [DOI] [PubMed] [Google Scholar]
- Gerber B, Seitz E, Muller H, Krause A, Reimer T, Kundt G, Friese K. Perioperative screening for metastatic disease is not indicated in patients with primary breast cancer and no clinical signs of tumor spread. Breast Cancer Res Treat. 2003;82:29–37. doi: 10.1023/B:BREA.0000003917.05413.ac. [DOI] [PubMed] [Google Scholar]
- Atalay G, Biganzoli L, Renard F, Paridaens R, Cufer T, Coleman R, Calvert AH, Gamucci T, Minisini A, Therasse P, Piccart MJ. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer. 2003;39:2439–2449. doi: 10.1016/s0959-8049(03)00601-4. [DOI] [PubMed] [Google Scholar]