Abstract
Interleukin-4 (IL-4) has been detected in both human and mouse atherosclerotic lesions, although its effects on the development of the disease are undefined. We determined the role of IL-4 in the most commonly used murine models of atherosclerosis by defining the effects of exogenous delivery and genetic deficiency of this cytokine on both hypercholesterolemia and AngII-induced atherosclerosis in apolipoprotein E (apoE)−/− mice and different dietary stimuli in low-density lipoprotein (LDL) receptor−/− mice. Exogenous administration of IL-4 (1.1 ng g−1 day−1 i.p. for 30 days) into female apoE−/− mice had no effect on lesion size or composition in mice fed normal or saturated fat diets. Also, IL-4 deficiency had no significant effect on the size or composition of atherosclerotic lesions in two vascular areas of male and female apoE−/− mice fed either a normal or saturated fat diet. IL-4 deficiency was also studied in age-matched male mice infused with AngII (1000 ng kg−1 min−1) for 28 days. Whereas AngII infusion augmented atherosclerotic lesion formation, IL-4 deficiency did not influence atherosclerotic lesion size or composition. Finally, different dietary stimuli also had no effect on atherosclerotic lesion size in female LDL receptor−/− mice. These data demonstrate that IL-4 does not significantly influence the development of atherosclerotic lesions in apoE−/− mice of either gender or in female LDL receptor−/− mice, irrespective of the mode of induction of atherosclerosis.
The principal infiltrating leukocytes in atherosclerotic lesions include monocyte-derived macrophages and T lymphocytes that serve as sources for secretion of many cytokines.1,2,3,4,5 Although there are many potential roles in which macrophages contribute to lesion formation, the role of T lymphocytes has yet to be clarified.6 An indication of an active role for T lymphocytes is provided by its presence throughout all stages of lesion development.3,5,7,8,9,10,11 CD4+ cells are the major class of T lymphocytes present in lesions. These T lymphocytes can be activated to differentiate into two major subsets, Th1 and Th2, that release a subset-specific repertoire of cytokines.
The predominance of studies have defined the role of Th1 cytokines on the development of atherosclerosis. These have included consistent demonstrations of the atherogenic properties of interferon-γ in apolipoprotein E (apoE)−/− and low-density lipoprotein (LDL) receptor−/− mice.12,13,14,15 Moreover, cytokines that mediate interferon-γ elaboration, including interleukin (IL)-1216 and IL-18,17,18,19 augment atherogenesis.
IL-4 is secreted by several cell types, including activated CD4+ T lymphocytes, mast cells, and a specialized subset of CD4+/natural killer cells, NK1.1+.20,21,22,23 IL-4 mRNA is present in atherosclerotic lesions from both mice and humans.11,24,25 Moreover, the abundance of IL-4 mRNA in atherosclerotic lesions increases in severely hypercholesterolemic states in apoE−/− mice.23 IL-4 has many potential effects on cultured vascular cells that could influence the atherogenic process, including up-regulation of CD36,26,27 class A scavenger receptors,28 matrix metalloproteinase 1,25 vascular cell adhesion molecule-1,29,30 monocyte chemoattractant protein-1,31 cysteinyl leukotriene 1 receptor,32 and 12/15 lipoxygenase.33,34 Conversely, IL-4 down-regulates inflammatory cytokine production and fibrinogen secretion in cultured vascular cells.35 Therefore, the overall effect of IL-4 on atherosclerosis is difficult to predict from these in vitro studies.
In addition to the many potential atherogenic effects that have been defined in cultured cells, a few studies have investigated the role of IL-4 on atherosclerosis in mouse models of the disease. Variable effects of IL-4 deficiency were noted on the small lesions induced in C57BL/6 mice by a variety of inflammatory stimuli.36,37 Also, site-specific reductions in lesion sizes were also noted in IL-4-deficient LDL receptor−/− mice that received bone marrow transplantation and were subsequently fed a cholate-containing diet.38 Finally, a single study of apoE−/− mice fed a normal diet demonstrated inconsistent effects of IL-4 deficiency on lesion size.16 Similar to results obtained from in vitro studies, studies investigating the effects of IL-4 deficiency in atherosclerosis have also been discordant. Therefore, the role of IL-4 in atherosclerosis requires further examination.
The aim of these studies was to comprehensively examine the effect of IL-4 on atherosclerotic lesion size and cellular composition. Because discrepancies in the literature regarding IL-4 in atherosclerosis may have arisen from differences in the experimental atherogenic diet, we examined lesion formation in LDL receptor−/− mice fed either a diet enriched in saturated fat and cholesterol or a cholate-containing atherogenic diet. Additionally, we examined lesion formation under a variety of conditions and used the two most commonly used models of atherosclerosis: apoE−/− and LDL receptor−/− mice.39,40 We examined the effects of exogenous delivery of IL-4, as well as the effects of deficiency of IL-4. In addition, we examined the effects of IL-4 deficiency on the induction of atherosclerosis by AngII.41 Furthermore, lesion sizes were quantified in two vascular areas. Under all these conditions, our results do not support a role for IL-4 in the modulation of atherosclerotic lesion size and composition in apoE−/− or LDL receptor−/− mice.
Materials and Methods
Animals
ApoE−/− (stock no. 2052), LDL receptor−/− (stock no. 2207), and IL-4−/− (stock no. 2253) mice, backcrossed 10 times onto a C57BL/6 background, were obtained from the Jackson Laboratory (Bar Harbor, ME). Both ApoE−/− and LDL receptor−/− mice were crossed into IL-4−/− mice to obtain homozygous compound-deficient mice. Mice were housed in a specific pathogen-free room and fed a normal diet (Ralston Purina, St. Louis, MO) before commencement of the study. Procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and conformed with the Guide for the Care and Use of Laboratory Animals published by the United States NIH (NIH Publication No. 85-23, revised 1996).
Genotyping and Phenotyping of Mice
PCR analysis of genomic DNA was performed to determine the genotype of the mice as described previously.38 Spleen cells were harvested as described previously.38 IL-4 production was determined in activated and nonactivated spleen cells using an IL-4 Cytotrap kit (BioSource, Camarillo, CA).
Diet-Induced Atherosclerosis Studies in LDL Receptor−/− Mice
Female LDL receptor−/− mice at 8 to 10 weeks of age that were either IL-4+/+ (n = 6 mice/group) or IL-4−/− (n = 9 mice/group) were fed either a diet enriched with 21% (w/w) butter fat and 0.15% (w/w) cholesterol (Teklad 88137; Teklad, Madison, WI) or a cholate-containing atherogenic diet with 21% (w/w) saturated fat, 1.25% (w/w) cholesterol, and 0.5% (w/w) cholate (Teklad 90221) for 28 days.
Exogenous IL-4 Administration Studies
Female apoE−/− mice (8 weeks of age; n = 10 mice/group) were placed on a diet enriched with 21% (w/w) fat and 0.15% (w/w) cholesterol (Teklad 88137) or a normal diet for 30 days. Recombinant mouse IL-4 (1.1 ng g−1 day−1; R&D Systems, Minneapolis, MN) or vehicle (0.1% bovine serum albumin in PBS) was administered daily by i.p. injection for 30 days.42
IL-4 Deficiency Studies
ApoE−/− male (n = 10 group) and female (n = 10 group) mice at 8 to 10 weeks of age that were either IL-4+/+ or IL-4−/− were fed either a normal diet until 36 weeks of age or a diet enriched with 21% (w/w) butter fat and 0.15% (w/w) cholesterol for 12 weeks.
AngII Infusion Studies
AngII (1000 ng kg−1 min−1; Sigma, St. Louis, MO) or sterile saline was infused via Alzet minipumps (Alzet, Cupertino, CA) for 28 days into 8-week-old male apoE−/− mice that were either IL-4+/+ (saline, n = 6; AngII, n = 8) or IL-4−/− (saline, n = 6; AngII, n = 8). Alzet minipumps were implanted subcutaneously on the right flank as described previously.43 Mice were fed a normal diet and water ad libitum throughout the study.38
Serum Lipids and Lipoprotein Distribution
Serum total cholesterol concentrations were determined using enzymatic assay kits (Wako Chemical Co., Richmond, VA). Lipoprotein cholesterol distributions were determined in individual serum samples (50 μl; n = 5 mice/group) as described previously.44
Atherosclerotic Lesion Analysis
Atherosclerosis was determined in both the aortic root and the aortic intimal surface as described previously.41,45
Immunostaining
For immunostaining, frozen tissue sections were initially incubated with serum from the species used to develop the secondary antibody. The primary antibodies used were rabbit mouse macrophage antiserum (1:3000 dilution) (AI-AD31240; Accurate Chemical, Westbury, NY) and a rat anti-mouse monoclonal T-lymphocyte IgG [Thy1.2 (1.67 μg/ml); PharMingen, San Diego, CA]. These antibodies were detected using an appropriate secondary biotinylated anti-rabbit or anti-rat IgG (1:200; Vector Laboratories, Burlingame, CA), respectively. Subsequently, tissues were incubated with a biotin-avidin-peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories). Immunoreactivity was visualized using the red chromagen 3-amino-9-ethyl carbazole (Biomeda Corp., Foster City, CA), and nuclei were counterstained with aqueous hematoxylin. Immunostained area of macrophage epitopes were quantified using ImagePro Software and normalized to percentage of atherosclerotic lesion area.
Statistics
Statistical analyses of studies were performed by Student’s t-test or by two-way analysis of variance, as appropriate. Significant interactions identified by analysis of variance were analyzed using a Tukey post hoc test for all pairwise comparisons. Nonparametric data were analyzed by Mann-Whitney rank sum test, or significant interactions were analyzed using a Holm-Sidak multiple comparison, where appropriate. All data analyses were performed using SigmaStat 3.0 software (SPSS, Inc., Chicago, IL) All data are represented as means ± SEM. P values <0.05 were considered to be statistically significant.
Results
IL-4 Deficiency in LDL Receptor−/− Mice
Previous studies from our laboratory demonstrated that transplantation of bone marrow from IL-4-deficient mice resulted in a modest decrease in atherosclerotic lesion size in the aortic root of female LDL receptor−/− mice fed a cholate-containing atherogenic diet.38 To define further the effects of IL-4 in atherogenesis, we fed female LDL receptor−/− mice that were either IL-4+/+ or IL-4−/− a diet enriched in saturated fat and cholesterol or a cholate-containing atherogenic diet for the 28-day time frame used in our previous bone marrow transplantation studies. Serum cholesterol concentrations (Table 1) were increased in mice of both genotypes fed the cholate-containing atherogenic diet. However, IL-4 deficiency did not alter serum cholesterol concentrations (Table 1) or serum lipoprotein distribution (data not shown) in mice fed either diet. Moreover, although atherosclerotic lesion burden was significantly increased in mice fed the cholate-containing atherogenic diet irrespective of genotype, lesion size was not affected by IL-4 deficiency in LDL receptor−/− mice fed either diet (Figure 1).
Table 1.
Effect of IL-4 Deficiency in LDL Receptor−/− Mice Fed a Cholate-Containing Atherogenic or Saturated Fat-Enriched Diet on Serum Cholesterol Concentrations
| Mice
|
Cholesterol concentrations (mg/dl)
|
|||
|---|---|---|---|---|
| IL-4 genotype | Sex | n | Cholate-containing atherogenic diet | Saturated fat-enriched diet |
| +/+ | Female | 6 | 1080 ± 52 | 661 ± 80 |
| −/− | Female | 9 | 1123 ± 51 | 600 ± 19 |
Data represent mean ± SEM. All intergroup comparisons for the same diet were not statistically significant.
Figure 1.
Atherosclerotic lesion area on the intimal surface of the aortic arch is not altered by IL-4 deficiency in LDL receptor-deficient mice fed a diet enriched in saturated fat that was either cholate-free (A) or supplemented (B). Eight- to 10-week-old female LDL receptor-deficient mice that were either IL-4+/+ or IL-4−/− were fed saturated fat-enriched diet (A) or cholate-containing atherogenic diet (B) for 28 days. After feeding of the diets, atherosclerotic lesions on the intimal surface of the aortic arch were measured in IL-4+/+ (□; n = 6 mice/group) and IL-4−/− (▪; n = 9 mice/group) mice at 9 to 11 weeks of age.
Exogenous Administration of Recombinant Mouse IL-4 in apoE−/− Mice
To investigate the effects of IL-4 on the atherogenic process in apoE−/− mice, recombinant murine IL-4 (1.1 ng g−1 day−1, i.p.) was administered to groups of age-matched females for a period of 30 days. This dose of IL-4 was demonstrated previously to prevent insulin-dependent diabetes mellitus in mice.42 Mice were fed either a normal or saturated fat-enriched diet during the 30 days of IL-4 administration. Exogenous administration of IL-4 increased systemic plasma concentrations of IL-4 in mice fed both diets [normal diet, 7.4 ± 1.9 (vehicle) versus 26.0 ± 7.2 pg/ml (IL-4); saturated fat-enriched diet, 6.9 ± 0.7 (vehicle) versus 16.9 ± 5.9 pg/ml (IL-4); P < 0.05 vehicle versus IL-4]. IL-4 administration did not alter body weight of mice fed either diet [normal diet, 19.2 ± 0.6 (vehicle) versus 19.2 ± 1.0 g (IL-4); saturated fat-enriched diet, 19.9 ± 1.0 (vehicle) versus 20.7 ± 0.8 g (IL-4)].
Exogenous administration of IL-4 did not alter serum cholesterol concentrations (Table 2) or serum lipoprotein distribution (data not shown) in mice fed either diet. Irrespective of diet, exogenous IL-4 administration had no effect on atherosclerotic lesion size in the aortic root (Figure 2, A and B) or on the luminal surface of the aortic arch (data not shown). No grossly discernable lesions were present in the thoracic and abdominal aortas of female mice fed either diet.
Table 2.
Effects of Exogenous IL-4 Administration or Deficiency on Serum Cholesterol Concentrations in apoE−/− Mice Fed either Normal or Saturated Fat-Enriched Diet
| Mice
|
Compound administered | Cholesterol concentrations (mg/dl)
|
|||
|---|---|---|---|---|---|
| IL-4 Genotype | Sex | n | Normal diet | Saturated fat-enriched diet | |
| +/+ | Female | 10 | Vehicle | 214 ± 8 | 530 ± 30 |
| 10 | IL-4 | 217 ± 5 | 498 ± 35 | ||
| +/+ | Female | 10 | None | 235 ± 16 | 520 ± 48 |
| Male | 10 | 215 ± 8 | 487 ± 26 | ||
| −/− | Female | 10 | 224 ± 13 | 508 ± 46 | |
| Male | 10 | 195 ± 10 | 496 ± 11 | ||
| +/+ | Male | 8 | Vehicle | 271 ± 12 | |
| Male | 8 | AngII | 240 ± 4 | ||
| −/− | Male | 8 | Vehicle | 221 ± 8 | |
| Male | 8 | AngII | 261 ± 25 | ||
Data represent mean ± SEM. All intergroup comparisons for the same diet were not statistically significant.
Figure 2.
Atherosclerotic lesion area in the aortic root is not altered by recombinant IL-4 administration in apoE−/− mice fed a normal (A) or saturated fat-enriched (B) diet. Eight-week-old female apoE−/− mice were fed either a normal or saturated fat-enriched diet for 30 days. Recombinant mouse IL-4 (1.1 ng g−1 day−1) or vehicle was administered daily by i.p. injection for a 30-day time course. Atherosclerotic lesions on the aortic root (A and B) were measured in vehicle (□) and recombinant IL-4-injected (▪) mice (n = 10 mice/group) at 9 weeks of age.
IL-4 Deficiency in apoE−/− Mice
Strain-matched apoE−/− mice that were either IL-4+/+ or IL-4−/− were generated to determine the effects of this cytokine on atherogenesis. PCR analyses of apoE and IL-4 were used to define the genotype of mice in the study and were confirmed by phenotyping with plasma lipid concentrations and IL-4 elaboration from isolated splenocytes, respectively.
Age- and gender-matched mice were fed either a normal or a saturated fat-enriched diet for 36 or 12 weeks, respectively. As expected, total serum cholesterol concentrations (Table 2) were increased in all mice fed the saturated fat-enriched diet. However, IL-4 deficiency did not alter serum concentrations of total cholesterol (Table 2) or lipoprotein distribution (data not shown) in either gender of apoE−/− mice fed normal or saturated fat-enriched diets. Analysis of the aortic root demonstrated no differences in atherosclerotic lesion formation in IL-4-deficient mice of either gender regardless of diet (Figure 3, A–D). Moreover, irrespective of diet, en face analysis of the entire luminal surface of the aorta demonstrated that IL-4 deficiency did not alter atherosclerotic lesion formation in apoE−/− mice (Figure 4, A–D). Whereas IL-4 deficiency did not alter lesion formation, gender had a significant effect on lesion formation in mice fed a normal diet through 36 weeks of age. Lesion formation both in the aortic root (Figure 3, A and B; P < 0.001) and on luminal surface of the aorta (Figure 4, A and B; P < 0.001) was significantly increased in female compared with male mice. Interestingly, this gender difference in lesion formation was not present in mice fed a saturated fat-enriched diet for 12 weeks.
Figure 3.
Atherosclerotic lesion area in the aortic root is not altered by IL-4 deficiency in apoE−/− mice fed either a normal or saturated fat-enriched diet. Atherosclerotic lesion in the aortic root were measured in male (A and C) and female (B and D) apoE−/− × IL-4+/+ (□) and apoE−/− × IL-4−/− (▪) mice fed a normal diet (A and B) until 36 weeks of age or a saturated fat-enriched diet (C and D) (n = 10 mice/group) for 12 weeks, beginning at 8 to 10 weeks of age (n = 10 mice/group).
Figure 4.
IL-4 deficiency does not alter atherosclerotic lesion area on the intimal surface of the aorta. Atherosclerotic lesions on the intimal surface of the aorta were measured in male (A and C) and female (B and D) apoE−/− × IL-4−/− (□) and apoE−/− × IL-4−/− (▪) mice fed either a normal diet (A and B) until 36 weeks of age or saturated fat-enriched diet (C and D) for 12 weeks, beginning at 8 to 10 weeks of age (n = 10 mice/group).
Immunostaining was performed on tissues from the aortic sinus. All lesions examined consisted predominantly of lipid-laden macrophages, with a small number of T lymphocytes. In addition to finding no effect of IL-4 deficiency on lesion size, we observed no overt differences in macrophage or T-lymphocyte content in lesions from either IL-4+/+ or IL-4IL-4−/− mice (Figure 5, A and B). Additionally, IL-4 deficiency did not alter the area of lesions that immunostained positively for macrophages in male and female mice fed either diet (Figure 5, C–F) [normal diet, 50 ± 13 (IL-4+/+ males) versus 48 ± 4% (IL-4−/− males); 50 ± 5 (IL-4+/+ females) versus 45 ± 2% (IL-4−/− females) macrophage epitope immunostained lesion area; saturated fat-enriched diet, 61 ± 2 (IL-4+/+ males) versus 63 ± 2% (IL-4−/− males); 60 ± 8 (IL-4+/+ females) versus 63 ± 4% (IL-4−/− females) macrophage epitope immunostained lesion area].
Figure 5.
IL-4 deficiency has no effect on cellular lesion composition. Immunostaining was performed using a rabbit antisera against mouse macrophages (A) (magnification, ×100) and an anti-rat monoclonal antibody against T lymphocytes (B) (Thy1.2; magnification, ×200) in representative sections of aortic root. Lesion area immunostained for macrophages was quantified in male and female mice fed a normal diet until 36 weeks of age (C and D) or saturated fat-enriched diet for 12 weeks (E and F), beginning at 8 to 10 weeks of age, and normalized to total lesion area.
IL-4 Deficiency in AngII-Induced Atherosclerosis in apoE−/− Mice
AngII infusion into hyperlipidemic mice accelerates the formation of atherosclerotic lesions, which are characterized by increased T-lymphocyte infiltration.41,46 The predominant source of IL-4 is T lymphocytes. Therefore, we sought to determine the role of IL-4 deficiency in AngII-induced atherogenesis.
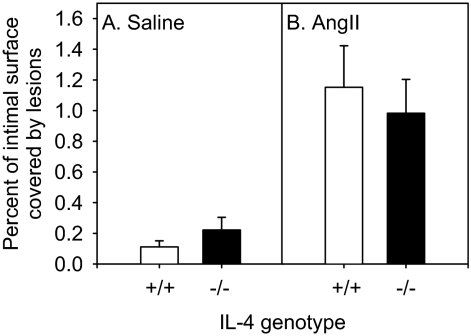
In agreement with previous studies, IL-4 deficiency did not alter serum cholesterol concentrations (Table 2) or lipoprotein distribution (data not shown) in mice infused with AngII (1000 ng kg−1 min−1) and fed a normal laboratory diet. The extent of atherosclerotic lesions was quantified on the intimal surface of the aortic arch. Irrespective of genotype, AngII infusion markedly enhanced atherosclerotic lesion formation (Figure 6). However, AngII-induced atherosclerotic lesion size on the aortic intimal surface was not altered by IL-4 deficiency in male apoE−/− mice (Figure 6).
Figure 6.
AngII-induced atherosclerotic lesion size in the aortic arch is not altered by IL-4 deficiency in apoE−/− mice. Atherosclerotic lesions on the intimal surface of the aortic arch were measured by en face analysis in 9-week-old male apoE−/− mice fed a normal diet and infused with saline (A) or AngII (B) (1000 ng kg−1 min−1) for 28 days that were IL-4+/+ (□) or IL-4−/− (▪; n = 8 mice/group).
Discussion
IL-4 has many effects on cultured cells that have been extrapolated to an effect on atherogenesis. Despite these in vitro studies, our results do not support a role of IL-4 as a modulator of the extent or composition of atherosclerotic lesions in apoE−/− or LDL receptor−/− mice that have not undergone bone marrow cell transplantation. Our studies were designed to determine the effects of IL-4 under multiple conditions. Although LDL receptor−/− mice have modestly increased plasma cholesterol concentrations on a normal diet, atherosclerotic lesion formation is not spontaneous and requires the feeding of saturated fat-enriched diets (reviewed in Refs. 47 and 48). In contrast, apoE−/− mice are hypercholesterolemic on normal diet and spontaneously develop atherosclerotic lesions. Diets enriched in saturated fat and cholesterol markedly increase cholesterol concentrations and accelerate the development of disease in apoE−/− mice.39,40 However, differences in atherosclerotic lesion formation have been noted in the response of different genes that are dependent on the composition of the diet fed to apoE−/− mice. For example, total lymphocyte deficiency due to disruption of the RAG genes decreased lesion size in mice fed a normal diet while having no effect on mice fed a saturated fat-enriched diet.49,50 Another rationale for using the different dietary stimuli was that IL-4 mRNA was only detected in mice that were rendered severely hypercholesterolemic, and thus IL-4 may have diet-specific effects.23 Despite these considerations, we were unable to detect an effect of IL-4 administration or deficiency on atherosclerotic lesion size and composition in either apoE−/− or LDL receptor−/− mice fed any of these experimental diets. We also examined the role of IL-4 in AngII-induced atherosclerosis, which has the potential to promote lesions through mechanisms that differ from hypercholesterolemia-induced disease.41,51 Again, we were unable to discern an effect of IL-4 on lesion size and composition.
A small number of studies have investigated the effects of IL-4 deficiency on lesion size. These include studies in wild-type C57BL/6 mice in which atherosclerotic lesion formation was promoted by feeding a diet enriched in saturated fat, cholesterol, and cholate or through the inflammatory stimuli of incomplete Freund’s adjuvant, heat shock protein-65, and Mycobacterium tuberculosis.36,37 IL-4 deficiency had no effect on the lesions formed during feeding of the cholate-containing diet or administration of incomplete Freund’s adjuvant. In contrast, IL-4 deficiency reduced the size of lesions formed by injected heat shock protein-65 or M. tuberculosis. The mechanism for the small lesions formed in aortic root of heat shock protein-65- and M. tuberculosis-injected mice is unknown. It is conceivable that although IL-4 plays no role in hypercholesterolemia or AngII-induced atherosclerosis, it may contribute to atherosclerosis generated by other differing mechanisms.
Compared with wild-type C57BL/6 mice, more substantial lesions of greater cellular complexity are formed in LDL receptor−/− and apoE−/− mice.52 Our previous study demonstrated that IL-4 deficiency reduced lesion size in LDL receptor−/− mice that had undergone bone marrow cell transplantation.38 However, this reduction was restricted to females, was modest, and only occurred in one vascular bed. Importantly, our previous study used a cholate-containing diet to promote atherosclerosis. It was subsequently realized that the inclusion of cholate may also have led to toxicity, as noted by a dramatic increase in gall stone formation and death.53 However, the present study demonstrates that IL-4 deficiency does not alter lesion size in LDL receptor−/− mice fed either a cholate-containing atherogenic or saturated fat-enriched diet. γ-Irradiation and bone marrow cell transplantation have been shown to alter lesion formation in LDL receptor−/− mice; however, these studies were performed in mice fed a non-cholate-containing atherogenic diet for 16 weeks.54 Therefore, extrapolation of these findings to explain the conflicting results between the present study and our previous bone marrow cell transplant study would not be a valid comparison based on the different time frames and dietary stimuli used in the studies. Given that our data demonstrating no effect of IL-4 deficiency in mice fed a cholate-containing diet agree with studies by George et al,36 these data may imply that bone marrow cell transplantation could have selectively affected lesion formation in IL-4-deficient mice.
There has also been a previous study of IL-4 deficiency in apoE−/− mice fed a normal diet.16 Variable effects were noted on lesion size depending on the vascular bed examined. These effects were dependent on site and time in an analysis that was performed on pooled genders. At 30 weeks of age, lesion size was reduced in the aortic root of IL-4-deficient mice with no effect in the aortic arch. At 45 weeks of age, there was no effect of IL-4 deficiency on lesion size in the aortic sinus, thorax, and abdominal aorta, but lesions were reduced in the arch. The present study also failed to demonstrate an effect of IL-4 deficiency on lesion size in the aortic arch in apoE−/− mice fed a normal diet for a similar interval. However, contrary to the findings of Davenport et al,16 we did not discern an effect of IL-4 deficiency on lesion size in the aortic sinus when the data were analyzed on a gender-specific basis. The contrasting findings between the two studies may be a result of the following: 1) apoE−/− mice used in these studies were obtained from different vendors. The present study used mice from the Jackson Laboratory, whereas Davenport et al16 used mice from Animal Resources Center (Canning Vale, Western Australia, Australia). 2) Lesion analysis in the root in the present study was performed over 400 mm2 area of the aortic sinus compared with 200 mm2 in the Davenport study. 3) En face analysis in the present study was represented as percent lesions on the entire intimal surface of the aorta, in contrast to the Davenport study, which represented data as regional areas. In agreement with the restricted conditions used by Davenport et al,16 we were also unable to detect changes in the size or composition of lesions from IL-4-deficient apoE−/− mice fed a saturated fat diet or infused with AngII.
Modulation of the helper T-lymphocyte (Th) balance toward a Th1 response promotes atherogenesis in mice.55 Extracts of human atherosclerotic lesions immunostain positive for Th1 cytokines, with little to no staining for Th2 cytokines, suggesting that the Th1 response also predominates in human atherosclerotic lesion formation.56 C57BL/6 mice mount predominately Th1 responses. Therefore, IL-4 deficiency may not have a profound effect in this background. However, increasing hypercholesterolemia is associated with a shift from a Th1 to a Th2 lymphocyte response in C57BL/6 apoE−/− mice.23 The shift from Th1 to Th2 responses occurred in mice with severe hypercholesterolemia (>1200 mg/dl). Moreover, mRNA for IL-4 was only detected in atherosclerotic lesions of mice with severe hypercholesterolemia (>1800 mg/dl) after feeding of a cholate-containing saturated fat-enriched diet.23 Although the apoE−/− mice in the present studies had hypercholesterolemia irrespective of the diet, the level of hypercholesterolemia (∼195 to 530 mg/dl) may not have been significant enough to drive an overwhelming Th1 to Th2 lymphocyte switch. Moreover, the saturated fat-enriched diet used in the apoE−/− mice did not contain cholate. Therefore, the lack of effect of IL-4 on atherogenesis in the present study may be due to the more moderate level of hypercholesterolemia in the apoE−/− mice compared with those observed in our previous study.38 Given the predominance of Th1 responses in C57BL/6 mice, the current study does not exclude the potential for an effect of IL-4 in other strains of mice.
A previous study demonstrated that exogenous administration of IL-4 into male C57BL/6 mice fed a cholate-containing diet decreased atherosclerotic lesion formation, which is associated with a reduction in interferon-γ.55 In contrast, we found no effect of exogenous IL-4 administration on lesion formation in female apoE−/− mice. Interferon-γ deficiency reduces atherosclerosis in male apoE−/− mice fed either a normal or a saturated fat-enriched diet; however, lesion formation is not altered in female apoE−/− mice.14 Given these data, we would not anticipate that reductions in interferon-γ mediated by IL-4 injection would alter atherosclerosis in female mice. Therefore, the gender difference in mice used in the present study, compared with the previous study, may account for the differing effects of IL-4 injection on atherogenesis. Moreover, differences in serum cholesterol concentrations, duration of the studies, and the concentrations of IL-4 administered exogenously may also contribute to the differing effects of IL-4 administration on atherosclerosis between the two studies. Additionally, although we found an increase in systemic plasma IL-4 concentrations after exogenous administration of IL-4 in the present study, this does not necessarily reflect the levels of IL-4 present in the vascular wall.
In conclusion, the present study determined the effects of IL-4 in LDL receptor−/− and apoE−/− mice using both exogenous administration and genetic deficiency in normal and two different high-fat diets in sex-, age-, and strain-matched groups. Furthermore, lesions were quantified and characterized in two vascular beds. The role of IL-4 was also studied in AngII-induced atherogenesis. Under these multiple circumstances, we were unable to determine a role for IL-4 in atherogenesis. These findings demonstrate that IL-4 does not effect atherosclerotic lesion formation in apoE−/− or LDL receptor−/− mice that have not undergone bone marrow cell transplantation.
Footnotes
Address reprint requests to Victoria L. King, Cardiovascular Research Center, Wethington Building, Room 562, University of Kentucky, Lexington, KY 40536-0200. E-mail: vicky.king@uky.edu.
Supported by the NIH (HL62846 and HL55487) and an American Heart Association (Ohio Valley Affiliate) Postdoctoral Fellowship award (AHA-0120324B to V.L.K.).
References
- Stemme S, Rymo L, Hansson GK. Polyclonal origin of lymphocytes-T in human atherosclerotic plaques. Lab Invest. 1991;65:654–660. [PubMed] [Google Scholar]
- Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Xu QB, Oberhuber G, Gruschwitz M, Wick G. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class-II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol. 1990;56:344–359. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]
- Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE−/− and LDL receptor−/− mice: decreasing density with disease progression. Arterioscler Thromb Vasc Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- Munro JM, Van der Walt JD, Munro CS, Chalmers JAC, Cox EL. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987;18:375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Hansson GK. Lymphocytes in atherogenesis. Dean RT, Kelly D, editors. Oxford, UK: Oxford Press,; Atherosclerosis. 2000:pp 230–249. [Google Scholar]
- Hansson GK, Jonasson L, Lojsthed B, Stemme S, Kocher O, Gabbiani G. Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis. 1988;72:135–141. doi: 10.1016/0021-9150(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- de Boer OJ, Van der Wal AC, Verhagen CE, Becker AE. Cytokine secretion profiles of cloned T cells from human aortic atherosclerotic plaques. J Pathol. 1999;188:174–179. doi: 10.1002/(SICI)1096-9896(199906)188:2<174::AID-PATH333>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kishikawa H, Shimokama T, Watanabe T. Localization of T lymphocytes and macrophages expressing IL-1, IL-2 receptor, IL-6 and TNF in human aortic intima: role of cell-mediated immunity in human atherogenesis. Virchows Archiv A Pathol Anat. 1993;423:433–442. doi: 10.1007/BF01606532. [DOI] [PubMed] [Google Scholar]
- Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Pablo AM, Jiang XC, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in apoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–1603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Paul WE. CD4pos, NK11pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XH, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti A, Kaveri S, Caligiuri G, Bariety J, Hansson GK. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. J Clin Invest. 1998;102:910–918. doi: 10.1172/JCI119892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri T, Arima N, Tanimoto A, Shimajiri S, Hamada T, Sasaguri Y. A role for interleukin 4 in production of matrix metalloproteinase 1 by human aortic smooth muscle cells. Atherosclerosis. 1998;138:247–253. doi: 10.1016/s0021-9150(97)00296-7. [DOI] [PubMed] [Google Scholar]
- Feng JW, Han JH, Pearce SFA, Silverstein RL, Gotto AM, Hajjar DP, Nicholson AC. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J Lipid Res. 2000;41:688–696. [PubMed] [Google Scholar]
- Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- Cornicelli JA, Butteiger D, Rateri DL, Welch K, Daugherty A. Interleukin-4 augments acetylated LDL induced cholesterol esterification in macrophages. J Lipid Res. 2000;41:376–383. [PubMed] [Google Scholar]
- Barks JL, McQuillan JJ, Iademarco MF. TNF-alpha and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J Immunol. 1997;159:4532–4538. [PubMed] [Google Scholar]
- Lee YW, Kuhn H, Hennig B, Neish AS, Toborek M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J Mol Cell Cardiol. 2001;33:83–94. doi: 10.1006/jmcc.2000.1278. [DOI] [PubMed] [Google Scholar]
- Walch L, Massade L, Dufilho M, Brunet A, Rendu F. Pro-atherogenic effect of interleukin-4 in endothelial cells: modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis. 2006;187:285–291. doi: 10.1016/j.atherosclerosis.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Woszczek G, Pawliczak R, Qi HY, Nagineni S, Alsaaty S, Logun C, Shelhamer JH. Functional characterization of human cysteinyl leukotriene 1 receptor gene structure. J Immunol. 2005;175:5152–5159. doi: 10.4049/jimmunol.175.8.5152. [DOI] [PubMed] [Google Scholar]
- Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci USA. 1992;89:217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Kuhn H, Kaiser S, Hennig B, Daugherty A, Toborek M. Interleukin 4 induces transcription of the 15-lipoxygenase I gene in human endothelial cells. J Lipid Res. 2001;42:783–791. [PubMed] [Google Scholar]
- Vasse M, Paysant I, Soria J, Mirshahi SS, Vannier JP, Soria C. Down-regulation of fibrinogen biosynthesis by IL-4, IL-10 and IL-13. Br J Haematol. 1996;93:955–961. doi: 10.1046/j.1365-2141.1996.d01-1731.x. [DOI] [PubMed] [Google Scholar]
- George J, Mulkins M, Shaish A, Casey S, Schatzman R, Sigal E, Harats D. Interleukin (IL)-4 deficiency does not influence fatty streak formation in C57BL/6 mice. Atherosclerosis. 2000;153:403–411. doi: 10.1016/s0021-9150(00)00418-4. [DOI] [PubMed] [Google Scholar]
- George J, Shoenfeld Y, Gilburd B, Afek A, Shaish A, Harats D. Requisite role for interleukin-4 in the acceleration of fatty streaks induced by heat shock protein 65 or Mycobacterium tuberculosis. Circ Res. 2000;86:1203–1210. doi: 10.1161/01.res.86.12.1203. [DOI] [PubMed] [Google Scholar]
- King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002;22:456–461. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aaltosetala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein-E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MJ, Arreaza GA, Zucker P, Chensue SW, Strieter RM, Chakrabarti S, Delovitch TL. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol. 1997;159:4686–4692. [PubMed] [Google Scholar]
- Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor−/− mice. Ann NY Acad Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- Sendobry SM, Cornicelli JA, Welch K, Bocan T, Tait B, Trivedi BK, Colbry N, Dyer RD, Feinmark SJ, Daugherty A. Attenuation of diet-induced atherosclerosis in rabbits with a highly selective 15-lipoxygenase inhibitor lacking significant antioxidant properties. Br J Pharmacol. 1997;120:1199–1206. doi: 10.1038/sj.bjp.0701007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- Wouters K, Shiri-Sverdlov R, van Gorp PJ, van Bilsen M, Hofker MH. Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoe and ldlr mice. Clin Chem Lab Med. 2005;43:470–479. doi: 10.1515/CCLM.2005.085. [DOI] [PubMed] [Google Scholar]
- Daugherty A. Mouse models of atherosclerosis. Am J Med Sci. 2002;323:3–10. doi: 10.1097/00000441-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA. 1997;94:4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A, Pure E, Delfel-Butteiger D, Chen S, Leferovich J, Roselaar SE, Rader DJ. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E−/− mice. J Clin Invest. 1997;100:1575–1580. doi: 10.1172/JCI119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in ApoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency promotes gallstone formation. J Lipid Res. 2002;43:768–771. [PubMed] [Google Scholar]
- Schiller NK, Kubo N, Boisvert WA, Curtiss LK. Effect of gamma-irradiation and bone marrow transplantation on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1674–1680. doi: 10.1161/hq1001.096724. [DOI] [PubMed] [Google Scholar]
- Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- Schönbeck U, Sukhova GK, Gerdes N, Libby P. T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Path. 2002;161:499–506. doi: 10.1016/S0002-9440(10)64206-X. [DOI] [PMC free article] [PubMed] [Google Scholar]