Abstract
De novo tissue generation stimulated by three angiogenic growth factors administered in a factorial design was studied in an in vivo murine tissue engineering chamber. A silicone chamber was implanted around the epigastric pedicle and filled with Matrigel with 100 ng/ml of recombinant mouse vascular endothelial growth factor-120 (VEGF120), recombinant human basic fibroblastic growth factor (FGF-2), or recombinant rat platelet-derived growth factor-BB (PDGF-BB) added as single, double, or triple combinations. Angiogenesis, supporting tissue ingrowth, and adipogenesis were assessed at 2 and 6 weeks by immunohistochemistry and morphometry. At 2 weeks angiogenesis was synergistically enhanced by VEGF120 + FGF-2 (P = 0.019). FGF-2 (P = 0.008) and PDGF-BB (P = 0.01) significantly increased connective tissue/inflammatory cell infiltrate (macrophages, pericytes, and preadipocytes) in double and triple combinations compared with control. At 6 weeks sequential addition of growth factors increased the percent volume of adipose tissue (P < 0.0005, each main effect), with a synergistic increase in adipose tissue in combination treatments (P < 0.0005). Groups containing 300 ng/ml of single growth factors produced significantly less adipose tissue than the triple growth factor combination (P < 0.0005, VEGF120 and PDGF-BB; P < 0.001, FGF-2). In conclusion, angiogenic growth factor combinations increased early angiogenesis and cell infiltration resulting in synergistically increased adipose tissue growth at 6 weeks. Two way and higher level synergies are likely to be important in therapeutic applications of angiogenic growth factors.
Adipose tissue displays angiogenic properties1,2 and has the capacity to continue growth throughout one’s lifespan with the potential to acquire new fat cells from fat cell precursors.3 Such increases in fat mass occur in tandem with an increase in the microcirculation.4 Angiogenesis and adipogenesis are tightly coordinated in both time and space during embryonic development.5 Observations in fetal pigs have demonstrated a positive correlation between fat cell density and capillary density in adipose tissue-forming areas.6
Neels and colleagues7 injected preadipocytes in nude mice and revealed increased expression of endothelial cell marker genes (CD31, Tie-1, and Tie-2) in parallel with an increase in adipogenic marker genes (lipoprotein, lipase, and adipsin) in developing fat pads. Dorsal skin fold injection of preadipocytes transfected with a dominant-negative PPAR-γ construct,8 known to inhibit adipogenesis9 not only abolished adipose formation but also reduced angiogenesis.8 Reciprocally, inhibition of angiogenesis with a vascular endothelial growth factor receptor 2 (VEGFR2)-blocking antibody reduced tissue growth and inhibited preadipocyte differentiation.8 Kawaguchi and colleagues10 also demonstrated that when Matrigel was implanted subdermally, supplemented with the angiogenic growth factor bFGF (FGF-2), neoangiogenesis was induced followed by adipocyte precursor recruitment.
A number of angiogenic growth factors acting at specific times in blood vessel development are known to play significant roles in angiogenesis. VEGF was first known as a vascular permeability factor11 but has since been recognized as being a potent stimulator of endothelial proliferation and migration12 and to induce the expression of interstitial collagenases.13 Fibroblastic growth factor (FGF)-2 is important in wound healing and angiogenesis, acting as an angiogenic cytokine promoting endothelial proliferation and capillary formation,14 and also stimulates proliferation and migration of mesenchymal cells.15 Platelet-derived growth factor (PDGF)-BB is involved in pericyte recruitment around capillaries during angiogenesis.16
Angiogenic growth factors have been used in tissue repair studies including those related to improving ischemic conditions. Single growth factor stimulation of neoangiogenesis has been disappointing.17 However, recent work with growth factor combinations—FGF-2 and PDGF-BB,18 VEGF-A and FGF-2,19,20 and VEGF-A and PDGF-BB21—have shown strong effects in promoting neoangiogenesis. Given the interdependence between angiogenesis and adipogenesis and the potential that efficiently engineered vascularized adipose tissue would have in tissue replacement procedures in breast, trauma, and cancer-related reconstructive procedures, this study explores the potential effect of single and combined therapies of VEGF120, PDGF-BB, and FGF-2 on angiogenesis and adipogenesis in an in vivo tissue-engineering model.
Materials and Methods
Animals
Experiments were approved by the St. Vincent’s Hospital Animal Ethics Committee, under National Health and Medical Research Council (Australia) guidelines. Wild-type male C57BL6 mice weighing 20 to 25 g were used. Surgical procedures were conducted under general anesthesia (chloral hydrate administered intraperitoneally at 0.4 mg/g body weight).
Matrigel and Growth Factors
Experimental groups are shown in Table 1. On the day of surgery, one, two, or three growth factors [recombinant human FGF-2 (CytoLab Ltd., Rehovot, Israel), and/or recombinant rat PDGF-BB (R&D Systems, Minneapolis, MN), and/or recombinant mouse VEGF120 (R&D Systems)] were added to growth factor-reduced Matrigel (BD Biosciences, Bedford, MA) as follows: 100 ng/ml Matrigel/growth factor in combination or separately were mixed with 80 U/ml of heparin (Pharmacia and Upjohn, Kalamazoo, MI). Matrigel was kept on ice before use to prevent it forming a gel. When the chamber was placed around the pedicle, 45 μl of growth factor/Matrigel solution was injected into the chamber and allowed to set for 2 minutes before the chamber was sealed.
Table 1.
Experimental Groups
| Group numbers* | Growth factor added | Time of harvest |
|---|---|---|
| 1 and 9 | None | (1) 2 weeks, (9) 6 weeks |
| 2 and 10 | 100 ng/ml VEGF120 | (2) 2 weeks, (10) 6 weeks |
| 3 and 11 | 100 ng/ml FGF-2 | (3) 2 weeks, (11) 6 weeks |
| 4 and 12 | 100 ng/ml PDGF-BB | (4) 2 weeks, (12) 6 weeks |
| 5 and 13 | 100 ng/ml VEGF120and 100 ng/ml PDGF-BB | (5) 2 weeks, (13) 6 weeks |
| 6 and14 | 100 ng/ml FGF-2 and 100 ng/ml PDGF-BB | (6) 2 weeks, (14) 6 weeks |
| 7 and 15 | 100 ng/ml VEGF120and 100 ng/ml FGF-2 | (7) 2 weeks, (15) 6 weeks |
| 8 and 16 | 100 ng/ml VEGF120, 100 ng/ml FGF-2, and 100 ng/ml PDGF-BB | (8) 2 weeks, (16) 6 weeks |
| 17 | 300 ng/ml VEGF120 | 6 weeks |
| 18 | 300 ng/ml FGF-2 | 6 weeks |
| 19 | 300 ng/ml PDGF-BB | 6 weeks |
n = 6 animals per group.
Growth factor concentrations at 100 ng/ml for the factorial study were chosen based on previous studies with FGF-2 in Matrigel10 indicating that 1000 ng/ml produced large amounts of adipose tissue, whereas 100 ng/ml produced less. Because we required a model in which the base line (single growth factor applications) produced some adipose tissue, but did not fill the chamber, and subsequent growth factor additions produced more adipose tissue, 100 ng/ml was selected as appropriate. In addition VEGF at 500 ng/ml and higher concentrations produces significant extravascular hemorrhage (G.M. Mitchell et al, unpublished observations), which we endeavored to avoid. Three additional growth factor groups were established in which a single growth factor (VEGF120, FGF-2, or PDGF-BB) at 300 ng/ml was added to chambers and explored at 6 weeks (Table 1).
Surgical Techniques
Cylindrical silicone chambers, 5 mm long, 3.35 mm internal diameter, 44 μl volume (Dow-Corning Corp., Midland, MI) were placed on the right epigastric pedicle according to a method modified from Cronin and colleagues22(Figure 1, a–d). The right superficial epigastric vessels were dissected free of surrounding tissue from their origin at the femoral vessels to their insertion into the groin fat pad. The cylindrical chamber was longitudinally cut and placed around the cleared epigastric vessels. The proximal femoral end and the lateral split were sealed with melted bone wax (Ethicon, Somerville, NJ). The chamber was then filled with Matrigel/growth factors. The distal end of the chamber was sealed with melted wax, without damaging the epigastric pedicle exiting the chamber. The skin wound was closed.
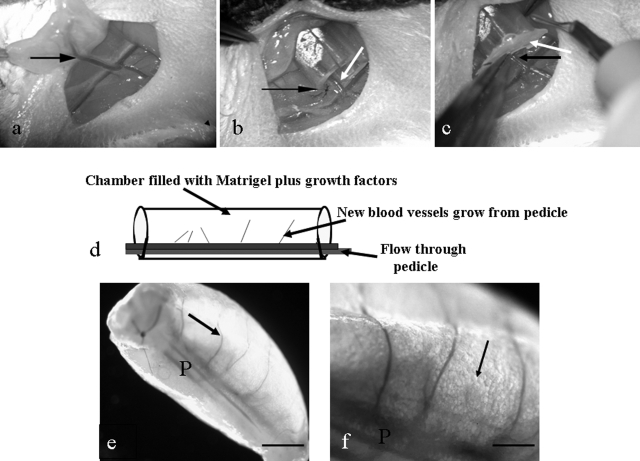
Figure 1.
a–c: Surgical procedure. a: Superficial inferior epigastric pedicle (arrow) branching off femoral vessels. b: Silicone chamber sutured into the groin (black arrow). Epigastric pedicle inside silicon chamber (white arrow). c: Bone wax sealing the base of the chamber (white arrow). Black arrow, epigastric pedicle entering chamber. d: Diagrammatic representation of the chamber construct. e and f: Macroscopic appearance of chamber construct at harvest. e: Growth factor-treated construct, after removal from the chamber. P, pedicle; arrow, new blood vessel. f: Higher magnification of the pedicle (P) demonstrating branches off the pedicle and spherical adipocytes (arrow). Scale bars: 1 mm (e); 0.5 mm (f).
Chamber Removal and Assessment
Animals were euthanized at 2 or 6 weeks after the construct was surgically explored, as indicated in Table 1. Pedicle patency was assessed by the presence or absence of bleeding from the cut pedicle. Chambers with occluded pedicles had no tissue growth and were replaced in the study, that is, new chambers were established with the appropriate growth factor combination. Construct volume was determined by displacement in a 0.9% NaCl solution.23 Tissue was fixed in 4% paraformaldehyde and embedded in paraffin. Five-μ-thick paraffin construct cross sections were stained with Masson’s trichrome for routine histological examination of all groups.
Immunohistochemistry: CD31, S-100, F4/80, α-SMA, and NG-2
Paraffin sections were dewaxed and hydrated and immunohistochemical staining performed using antibodies directed against CD31 (mouse endothelial cell marker), S-100 (preadipocyte/adipocyte marker), F4/80 (mouse macrophage marker), α-smooth muscle actin (α-SMA), and NG-2—chondroitin sulfate proteoglycan (pericyte marker). Reagents were purchased from DAKO (Carpinteria, CA) unless otherwise stated. Positive control tissue of known staining pattern and intensity was included in each run to ensure consistency between runs. For negative controls, diluent alone or nonimmune immunoglobulin at the same concentration was substituted for the primary antibody. Sections were counterstained with hematoxylin.
For CD31 (rat anti-mouse CD31; BD Pharmingen, San Jose, CA), sections were digested with DAKO proteinase K for 8 minutes and nonspecific binding blocked using DAKO protein block for 30 minutes. The primary antibody was applied at 1:150 in DAKO antibody diluent for 1 hour, followed by the secondary antibody (DAKO rabbit anti-rat biotinylated immunoglobulin) at 1:300 for 30 minutes. An avidin-biotin-horseradish peroxidase detection system (ABC Elite; Vector Laboratories, Burlingame, CA) was used for 30 minutes, followed by diaminobenzidine (DAB) color development.
S-100 (rabbit anti-cow S-100) antigen retrieval was performed using a 10 mmol/L ethylenediaminetetraacetic acid, pH 8.0, buffer in a 95°C water bath for 30 minutes, followed by 30 minutes of cool-down. Sections were blocked in 10% normal swine serum for 30 minutes, and primary antibody was applied at 1:1000 overnight at 4°C, followed by swine anti-rabbit biotin at 1:200 for 30 minutes, horseradish peroxidase-streptavidin at 1:400 for 30 minutes, and DAB.
For F4/80 (rat anti-mouse F4/80; Abcam, Cambridge UK) sections were digested with proteinase K for 3 minutes and blocked with 10% normal swine serum for 30 minutes. Primary antibody was applied (1:500 in 5% normal rabbit serum for 60 minutes), followed by rabbit anti-rat biotin (1:200 for 30 minutes), horseradish peroxidase-streptavidin (1:400 for 30 minutes), and DAB.
For smooth muscle actin (mouse anti-human smooth muscle actin, clone 1A4), primary antibody was applied (1:400 for 30 minutes), followed by DAKO mouse envision for 30 minutes and DAB.
For NG-2 (rabbit anti-rat NG-2 chondroitin sulfate proteoglycan; Chemicon, Temecula, CA) the retrieval protocol was as for S-100. Sections were blocked with DAKO protein block for 15 minutes and then primary antibody (1:100 for 60 minutes), followed by goat anti-rabbit biotin (1:300 for 30 minutes, Vector Laboratories), horseradish peroxidase-streptavidin (1:400 for 30 minutes), and DAB.
Morphometric Assessment
Percent Volumes
The percent vascular volume (PVV) and percent volume of connective tissue/inflammatory cell infiltrate at 2 weeks (n = 3–5/group) and percent adipose tissue volume and PVV at 6 weeks (n = 6/group) was determined on CD31/hematoxylin-labeled slides, by point counting,24,25 with an observer blinded to the group identity. At least two cross sections of each chamber (500 μm apart) were counted on an Olympus (Tokyo, Japan) microscope. Using digital video imaging (TK C 1480E; JVC, Wayne, NJ), an automated systematic random sampling point-counting system was applied using the Computer Assisted Stereological Toolbox (CAST System; Olympus, Ballerup, Denmark). The 2-week PVV was multiplied by the total area of tissue growth in construct cross sections. This parameter measured the total area of all new vasculature at 2 weeks.
Experimental Design and Statistical Analysis
The three treatment applications: VEGF120, FGF-2, and PDGF-BB were combined factorially to give eight experimental groups including a control group without growth factors at two time points (2 and 6 weeks), therefore a total of 16 groups were established. The factorial design allowed a comparison by three-way analysis of variance on SPSS for Windows (version 12.0.1; SPSS Corporation, Chicago, IL). Comparisons were made for the main effect of each treatment across all animals in the study as well as assessing interactions between each treatment. Synergy between treatments was assessed by significance in the measures of interaction. α was set at 0.05. For the comparison of 300-ng/ml groups of single growth factors to the triple growth factor group at 6 weeks, a one-way analysis of variance was conducted and Dunnett’s t-test applied to compare all groups against the triple growth factor group.
Results
Macroscopic Appearance
At 2 and 6 weeks new vascular branches sprouting off the epigastric pedicle were visible running along one side of the construct (Figure 1, e and f). At 2 weeks new tissue infiltration had not filled the Matrigel, whereas at 6 weeks tissue infiltration was more advanced, and numerous small spheres were observed around new blood vessels that were assumed to be adipocytes (Figure 1f). At 6 weeks constructs were 4 to 5 mm long and 2 to 3 mm wide, and construct volume did not differ between groups.
Morphological Assessment
Two-Week Chambers
The pedicle (epigastric artery, vein, and nerve) was observed at one side of the construct with immature new blood vessels and numerous other cells (mesenchymal cells and leukocytes) radiating from the pedicle (Figure 2, a and b). A cuff of empty Matrigel was observed in the area farthest from the pedicle. The capsule, partially encompassing the construct originated from the pedicle area. The cellularity of the invading tissue increased around the pedicle and new capillary sprouts in double and triple growth factor combinations compared with control (Figure 2c), being particularly apparent in the VEGF120 + FGF-2 and the triple growth factor combination groups (Figure 2, d and e). Neutrophils and fibroblast-like cells were readily identified in this cellular infiltration.
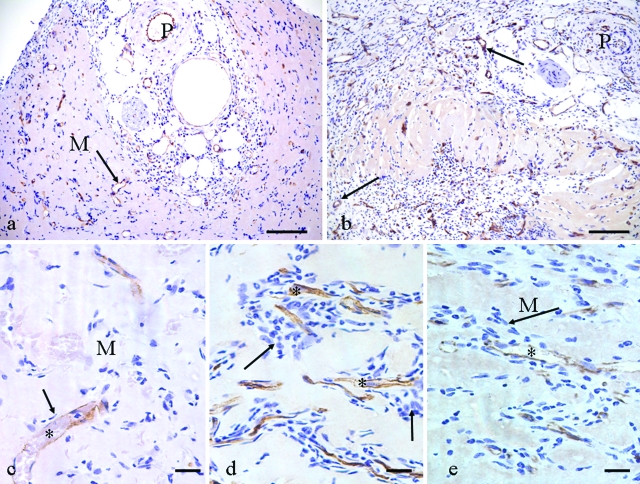
Figure 2.
CD31-labeled tissue, 2 weeks. a: Control, pedicle cross section (P) and new blood vessels (arrow) radiating into empty Matrigel (M). b: Triple growth factor treated, demonstrating a general increase in cellularity and many more new blood vessels (arrows) compared with control. P, pedicle. c: Vascular sprouts in control. Arrow indicates few accompanying cells with a new capillary (*) in relatively empty Matrigel (M). d and e: VEGF120 + FGF-2 group (d) and triple growth factor group (e), both demonstrate increased cellularity (arrows) around CD31-positive capillary sprouts (*). M, empty Matrigel. Compare with c. Scale bars: 100 μm (a, b); 20 μm (c–e).
Immunohistochemical Identification of Cells Accompanying Capillary Sprouts: S-100 labeled numerous connective tissue-like cells in capsule and pedicle regions in treated and control samples. Some, but not all, triple growth factor-treated specimens displayed increased numbers of S-100-positive cells around capillaries (Figure 3, a–d) compared with control. Macrophages (F4/80-positive) were numerous in controls but more numerous in the triple growth factor group. This was particularly obvious around capillary sprouts (Figure 3, e–h).
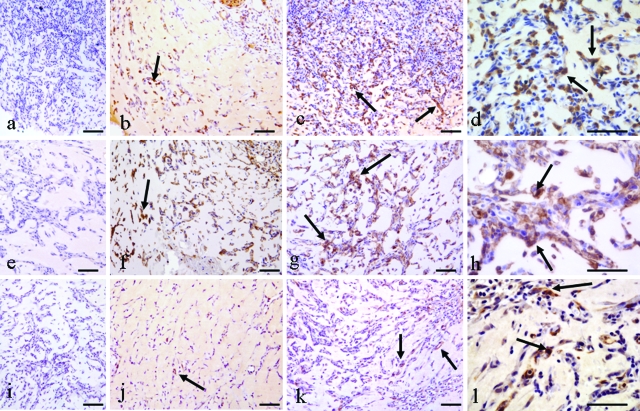
Figure 3.
Immunohistochemical identification of cell populations around capillary sprouts at 2 weeks. a–d: S-100 labeling. a: Negative control. b: Control, some S-100-positive cells (arrow). c: Triple growth factor treated demonstrates many more S-100-positive cells (arrows). d: Higher power of c demonstrating numerous S-100-positive cells (arrows). e–h: F4/80 labeling. e: Negative control. f: Control, some F4/80-positive cells (arrows). g: Triple growth factor treated with larger numbers of F4/80-positive cells (arrows) than control. h: Higher power of c demonstrating large numbers of macrophages (arrows) around capillary sprouts. i–l: NG-2 labeling. i: Negative control. j: Control, demonstrates a small number of NG-2-positive cells (arrow). k: Triple growth factor treated, demonstrating more NG-2-positive cells (arrows) than control. l: Higher power of c, NG-2-positive cells are numerous (arrows). Scale bars: 100 μm (a–c, e–g, i–k); 50 μm (d, h, l).
α-SMA labeling occurred in capsule myofibroblasts and in smooth muscle cells of pedicle vessels and larger new blood vessels, and no difference was evident between control and growth factor-treated samples (data not shown). NG-2 labeling was observed in capsular and pedicle regions of control and growth factor-treated constructs. There was an increased incidence around capillaries in the triple growth factor group compared with controls (Figure 3, i–l). Thus the double and triple growth factor groups demonstrated a marked increase in cellularity at 2 weeks. The increased cell populations as-sociated with new capillaries included neutrophils, S-100-positive preadipocytes (variable), F4/80-positive macrophages, and NG-2-positive pericytes.
Six-Week Chambers
In cross section all constructs were completely surrounded by a well vascularized connective tissue capsule, the thickness of which varied according to growth factor treatment, being thickest with PDGF-BB treatment. Mature blood vessels radiated from the patent pedicle artery and vein into surrounding tissue (Figure 4, a and b). Blood vessels also extended into the Matrigel from the vascular capsule. Immature adipocytes of various sizes demonstrating multilocular cytoplasm were frequently observed adjacent to blood vessels in distant parts of the construct (Figure 4, c and d). The density of adipose tissue formation depended on the growth factor treatment. In control tissue there was rare adipocyte development (Figure 4c). Mature and immature adipocyte cell numbers increased in single growth factor treatment regimes except the VEGF120 group. Double and particularly triple growth factor regimes increased adipose tissue development further (Figure 4d, and morphometry section). Capillaries were often seen in close association with developing adipocytes (Figure 4, b–d). Groups with 300 ng/ml of a single growth factor all demonstrated a similar general construct morphology to the other 6-week groups. Adipose tissue development occurred in all three groups and was most pronounced in the FGF-2 group.
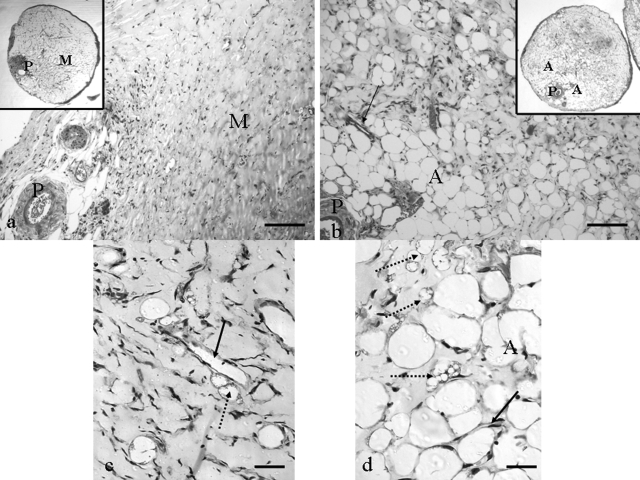
Figure 4.
Adipose tissue growth at 6 weeks (5-μm-thick paraffin sections, Masson’s trichrome stained). a: Control, demonstrating pedicle (P) and limited connective tissue ingrowth into the Matrigel (M). No adipose tissue is evident. b: Triple growth factor treated, demonstrating large areas of well vascularized (arrow) adipose tissue (A) around pedicle (P). Insets in a and b are low-power images of full cross sections of control and triple growth factor-treated constructs. c: Higher power of control. Immature adipocytes (dotted arrow) are seen occasionally adjacent to a capillary (solid arrow). Mature adipocytes are rarely observed. d: Higher power of a triple growth factor-treated chamber with large numbers of mature adipocytes (A), many immature adipocytes (dotted arrows) and capillaries (solid arrow). Scale bars: 100 μm (a, b); 20 μm (c, d).
Morphometry
Two Weeks
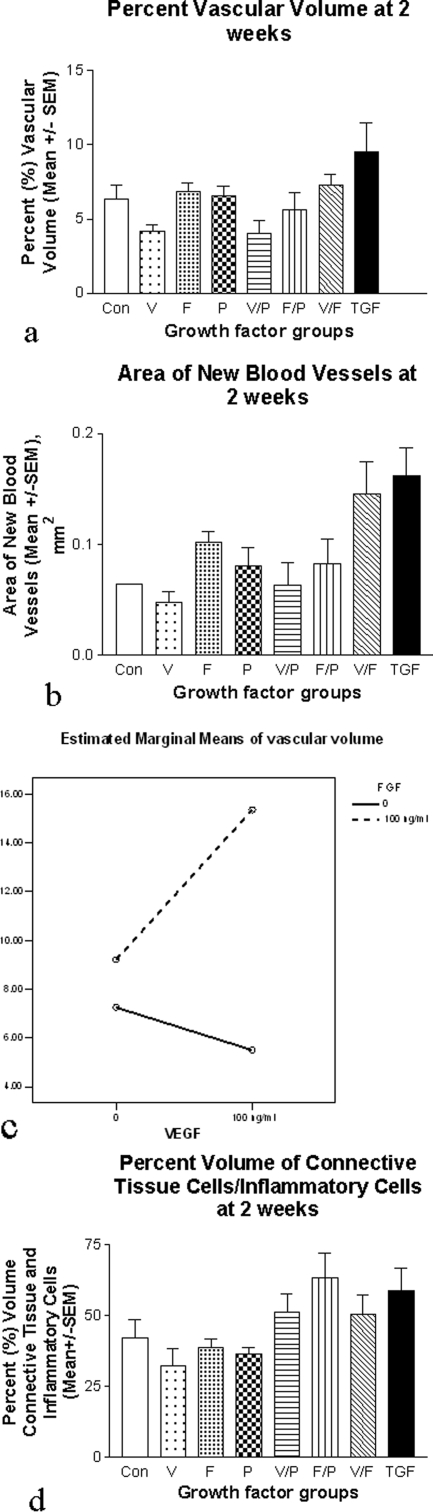
Vascular Tissue: The triple growth factor group demonstrated the highest PVV (9.5 ± 1.9%) compared with control (6.3 ± 0.96%, Figure 5a). Total new blood vessel area was markedly increased in the VEGF120 + FGF-2 (0.146 ± 0.29 mm2) and triple growth factor groups (0.16 ± 0.25 mm2), compared with control (0.06 ± 0.00005 mm2, Figure 5b). FGF-2 was found to significantly increase the vasculature (P < 0.001), and there was a significant interaction (synergy) between FGF-2 and VEGF120, (P = 0.019, Figure 5c).
Figure 5.
Two-week morphometry. a: Triple growth factor group demonstrates highest PVV at 2 weeks. b: VEGF120 + FGF-2 and the triple growth factor combination groups demonstrate increased new vascular cross-sectional area at 2 weeks. c: A significant positive interaction was seen between FGF-2 and VEGF in generation of vascular tissue (P = 0.019). d: Percent volume of connective tissue/inflammatory components at 2 weeks, demonstrating increases in all double and triple combinations. FGF-2 (P = 0.008) and PDGF-BB (P = 0.01) significantly increased this component.
Connective Tissue: Two or more growth factors increased the connective tissue/inflammatory infiltrate, regardless of combination, compared with control and single growth factor administration (Figure 5d). Both FGF-2 (P = 0.008) and PDGF-BB (P = 0.01) significantly increased this component. There were no significant interactions.
Six Weeks
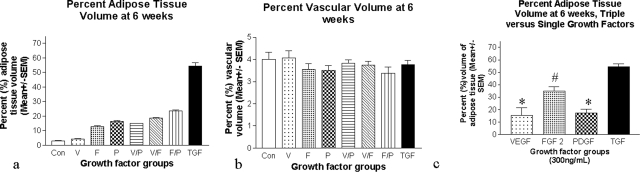
Adipose Tissue: At 6 weeks the single addition of each growth factor resulted in an increase in the percent adipose tissue volume in the construct (VEGF120, 4.18 ± 0.32%; FGF-2,: 12.72 ± 0.83%; PDGF-BB, 16.44 ± 0.0.39%) compared with control (3.00 ± 0.16%, Figure 6a), although the increase was minor for VEGF120.
Figure 6.
Six-week morphometry. a: Percent volume of adipose tissue at 6 weeks gradually increases as the number of angiogenic growth factors used increases, with the triple growth factor group demonstrating a marked increase over all other groups. Synergy existed between the three growth factors in fat production (P value for overall interaction of VEGF120, PDGF-BB, and FGF-2 <0.0005). b: PVV at 6 weeks. There were no significant differences between groups. c: The percent volume of adipose tissue at 6 weeks in the triple growth factor group (total growth factors = 300 ng/ml) was significantly elevated compared with single growth factors administered at 300 ng/ml. *P < 0.0005 for triple growth factor comparison with VEGF120 and PDGF-BB, and #P < 0.001 for triple growth factor comparison with FGF-2.
Double growth factor combinations increased the percent adipose tissue volume further (VEGF120 + FGF-2, 18.50 ± 0.53%; VEGF120 + PDGF-BB, 15.14 ± 0.97%; and FGF-2 + PDGF-BB, 23.44 ± 0.74%; Figure 6a) and the triple growth factor group even more (54.00 ± 2.30%). The increase in fat attributable to the addition of each growth factor was in each case highly significant (P < 0.0005 for each main effect).
There was strong evidence of synergy between the three growth factors in fat production (P value for overall interaction of VEGF120, PDGF-BB, and FGF-2 < 0.0005). VEGF120 + PDGF-BB had a significant positive interaction (P < 0.0005) as did VEGF120 + FGF-2 (P = 0.0005) and PDGF-BB + FGF-2 (P = 0.0005). There was also a significantly increased adipose tissue production in the triple growth factor group (300 ng/ml total growth factors) compared with groups receiving 300 ng/ml of a single growth factor (P < 0.0005 for comparison with VEGF120, PDGF-BB, and P < 0.001 for comparison with FGF-2, Figure 6c).
Vascular Tissue: No difference in percent blood vessel volume was detected between groups at 6 weeks (Figure 6c). It was noted that PVV at 6 weeks was similar to or less than at 2 weeks for most groups (compare Figure 5a with Figure 6b).
Discussion
In a murine in vivo tissue engineering chamber model the addition of VEGF120, FGF-2, and PDGF-BB in a factorial design induced significant and contrasting responses at 2 and 6 weeks. At 2 weeks VEGF120 + FGF-2 synergistically increased angiogenesis, whereas all double and triple growth factor combinations demonstrated increases in connective tissue/inflammatory cell infiltrate, which included neutrophils, macrophages, preadipocytes, and pericytes. FGF-2 and PDGF-BB both significantly increased this component at 2 weeks. At 6 weeks differences in vascularization were no longer apparent, but all three angiogenic growth factor combinations (VEGF120 + FGF-2, VEGF120 + PDGF-BB, FGF-2 + PDGF-BB) synergistically increased adipose tissue formation. This synergistic increase in adipose tissue production by combination treatments was further validated by single growth factor applications at 300 ng/ml, all of which produced significantly less adipose tissue (P < 0.0005 for VEGF120 and PDGF-BB, P < 0.001 for FGF-2) than the triple growth factor group, indicating that total growth factor load is not the only factor involved in adipose tissue production in this model.
Applications of angiogenic growth factors in models of adipogenesis and angiogenesis have been previously described. Basic fibroblastic growth factor (FGF-2) is thought to be a critical factor both in adipose development and adipose-dependent angiogenesis26 and has been demonstrated to result in de novo adipogenesis when combined with Matrigel in an in vivo tissue-engineering construct.10,27,28 Similarly, recombinant platelet-derived growth factor was shown to generate neovascularized soft tissue appendages when bound to a collagen matrix29 and in in vitro studies to promote preadipocyte differentiation.30 As previously discussed, although VEGF does not directly affect adipocyte differentiation, VEGFR2 signaling increases preadipocyte survival and differentiation in a paracrine manner.8
Significantly increased adipose tissue production in growth factor combination groups, particularly the triple growth factor combination, may result from early increased angiogenesis, demonstrated in this and other studies,18,19,20,21 and accompanying early increased infiltration of mesenchymal progenitor cells. Recent studies suggest that blood-borne fibrocytes leave the circulation and differentiate into adipocytes.31 The up-regulation of PDGFRβ receptors,19 and enhanced migration of mesenchymal cells carrying these receptors and the possibility that PDGF may enhance murine preadipocyte differentiation,30 may explain the synergistic increase in adipogenesis.
A stimulatory effect on angiogenesis and early tissue migration and proliferation by double and triple growth factor regimes is supported in this study by the observed increases in connective tissue/inflammatory cell infiltrate at 2 weeks involving increased macrophage, pericyte, and (variably) preadipocyte populations. Both macrophages and pericytes have been implicated in angiogenesis16,32 and adipogenesis.33,34 The cellular infiltration around capillary sprouts is where immature adipocytes occur at 6 weeks and has been observed by others.32
PDGF-BB and FGF-2 stimulate mesenchymal cell proliferation and migration,15,29,35,36,37 PDGF-BB stimulates preadipocyte differentiation,30 whereas FGF-2 stimulates proliferation of adipose precursor cells.38 Given the evidence that both factors, via several mechanisms, positively influence adipose tissue growth, it is not surprising that our study showed an increase in adipose tissue for individual applications of FGF-2 and PDGF-BB. Interactions between growth factors resulted in significant increases in percent adipose tissue at 6 weeks. These synergistic effects on adipose tissue production [P < 0.0005 for overall interaction of VEGF120, FGF-2, PDGF-BB, and VEGF120 + PDGF-BB (P < 0.0005), VEGF120 + FGF-2 (P = 0.0005), PDGF-BB + FGF-2 (P = 0.0005)] have not been reported previously.
Thus in summary, evidence from our model suggests that synergistically enhanced angiogenesis at 2 weeks with VEGF 120 + FGF-2 may also promote PDGF-B-PDGFRB signaling19 that further enhances already increased numbers of mesenchymal cells induced by FGF-2. This attraction of mesenchymal precursors may be further enhanced when we additionally administer PDGF-BB, favoring the further attraction and differentiation of adipocyte precursors in the favorable chamber environment. These are the likely mechanisms involved in a synergistic increase in adipose tissue development with the triple growth factor regime.
The PVV at 6 weeks did not differ between groups and had declined in some groups at 6 weeks compared with 2 weeks. It appears from our study that the synergistic increase in angiogenesis at 2 weeks provided by the VEGF120 + FGF-2 combination is transitory in this model and that subsequent remodeling occurs as adipose tissue formation takes place. Decreases in vascular volume and blood vessel diameter have been reported previously in developing fat pads after 14 days.8 Similarly, a recent publication39 has revealed remodeling in other highly vascular tissue engineered microcirculatory networks from ∼3 weeks. Our results are consistent with these previous studies.
This tissue engineering chamber model of angiogenic and adipogenic induction confines the construct inside a protected space and isolates Matrigel from the surrounding subcutaneous fat preventing direct migration of preadipocytes from adjacent tissue. Our model therefore differs from previous successful adipogenic models.10,22 Recent studies in our model confirm that new adipose tissue is derived from host cells, systemically derived,40 in line with other recent studies.31
The addition of growth factor combinations of VEGF120, FGF-2, and PDGF-BB increased not only early angiogenesis but also the migration into the chamber construct of blood-borne adipogenic cell populations that exponentially increased adipose tissue formation. However, if the chamber vascular pedicle is occluded, no adipose tissue forms. Thus, these angiogenic growth factor combinations have synergistically increased tissue growth other than that of the vasculature, indicating significant therapeutic advantages of this protocol in the field of regenerative medicine.
Acknowledgments
We thank the staff of the Experimental and Medical Surgical Unit at St. Vincent’s Hospital, Melbourne, for their assistance in animal surgical procedures.
Footnotes
Address reprint requests to Dr. Geraldine Mitchell, Ph.D., Bernard O’Brien Institute of Microsurgery, 42 Fitzroy St., Fitzroy, Victoria, 3065, Australia. E-mail: geraldine.mitchell@svhm.org.au or mitcg@unimelb.edu.au.
Supported by the National Health and Medical Research Council of Australia (postgraduate scholarship to J.A.R.).
References
- Silverman KJ, Lund DP, Zetter BR, Lainey LL, Shahood JA, Freiman DG, Folkman J, Barger AC. Angiogenic activity of adipose tissue. Biochem Biophys Res Commun. 1988;153:347–352. doi: 10.1016/s0006-291x(88)81229-4. [DOI] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Lolmede K, Sengenes C, Galitzky J, Lafontan M. Angiogenesis in adipose tissue. Ann Endocrinol (Paris) 2002;63:91–95. [PubMed] [Google Scholar]
- Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- Hausman GJ, Thomas GB. Structural and histochemical aspects of perirenal adipose tissue in fetal pigs: relationships between stromal-vascular characteristics and fat cell concentration and enzyme activity. J Morphol. 1986;190:271–283. doi: 10.1002/jmor.1051900304. [DOI] [PubMed] [Google Scholar]
- Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 2004;18:983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–e97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS. PPARgamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis. Genes Dev. 2002;16:27–32. doi: 10.1101/gad.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA. 1998;95:1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF, Dvorak AM, Manseau EJ, Wiberg L, Churchill WH. Fibrin gel investment associated with line 1 and 10 solid tumor growth, angiogenesis, and fibroplasia in guinea pigs. Role of cellular immunity, myofibroblasts, microvascular damage, and infarction in line 1 tumor regression. J Natl Cancer Inst. 1979;62:1459–1472. [PubMed] [Google Scholar]
- Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992;153:557–562. doi: 10.1002/jcp.1041530317. [DOI] [PubMed] [Google Scholar]
- Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Chiou M, Xu Y, Longaker M. Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochem Biophys Res Commun. 2006;343:644–652. doi: 10.1016/j.bbrc.2006.02.171. [DOI] [PubMed] [Google Scholar]
- Darland DC, D’Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103:157–158. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, Miyazono K, Miyazawa K. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci. 2005;118:3759–3768. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- Ley C, Olsen M, Lund E, Kristjansen P. Angiogenic synergy of bFGF and VEGF is antagonized by angiopoietin-2 in a modified in vivo Matrigel assay. Microvasc Res. 2004;68:161–168. doi: 10.1016/j.mvr.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- Cronin KJ, Messina A, Knight KR, Cooper-White JJ, Stevens GW, Penington AJ, Morrison WA. New murine model of spontaneous autologous tissue engineering, combining an arteriovenous pedicle with matrix materials. Plast Reconstr Surg. 2004;113:260–269. doi: 10.1097/01.PRS.0000095942.71618.9D. [DOI] [PubMed] [Google Scholar]
- Scherle W. A simple method for volumetry of organs in qualitative sterology. Mikroskopie. 1970;26:57–60.. [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology. Abington: Bios Scientific Publishers,; 1998 [Google Scholar]
- Lokmic Z, Darby IA, Thompson EW, Mitchell GM. A time course of hypoxia, granulation tissue and blood vessel growth and remodelling in healing rat cutaneous incisional primary intention wounds. Wound Repair Regen. 2006;14:277–288. doi: 10.1111/j.1743-6109.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Control of angiogenesis by heparin and other sulfated polysaccharides. Adv Exp Med Biol. 1992;313:355–364. doi: 10.1007/978-1-4899-2444-5_34. [DOI] [PubMed] [Google Scholar]
- Tabata Y, Miyao M, Inamoto T, Ishii T, Hirano Y, Yamaoki Y, Ikada Y. De novo formation of adipose tissue by controlled release of basic fibroblast growth factor. Tissue Eng. 2000;6:279–289. doi: 10.1089/10763270050044452. [DOI] [PubMed] [Google Scholar]
- Walton RL, Beahm EK, Wu L. De novo adipose formation in a vascularized engineered construct. Microsurgery. 2004;24:378–384. doi: 10.1002/micr.20056. [DOI] [PubMed] [Google Scholar]
- Khouri RK, Koudsi B, Deune EG, Hong SP, Ozbek MR, Serdar CM, Song SZ, Pierce GF. Tissue generation with growth factors. Surgery. 1993;114:374–380. [PubMed] [Google Scholar]
- Bachmeier M, Loffler G. Influence of growth factors on growth and differentiation of 3T3–L1 preadipocytes in serum-free conditions. Eur J Cell Biol. 1995;68:323–329. [PubMed] [Google Scholar]
- Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029–2031. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol. 2006;168:529–541. doi: 10.2353/ajpath.2006.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Poucher SM, Lu J, Henry PD. Fibroblast growth factor 2: from laboratory evidence to clinical application. Curr Vasc Pharmacol. 2004;2:33–43. doi: 10.2174/1570161043476500. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tajima R, Tsutsumi A, Akamatasu J, Ohba S. New matrix flap prefabricated by arteriovenous shunting and artificial skin dermis in rats. II. Effect of interpositional vein and artery grafts and bFGF on new tissue generation. J Jpn Plast Reconstr Surg. 1996;16:679–686.. [Google Scholar]
- Claffey KP, Abrams K, Shih SC, Brown LF, Mullen A, Keough M. Fibroblast growth factor 2 activation of stromal cell vascular endothelial growth factor expression and angiogenesis. Lab Invest. 2001;81:61–75. doi: 10.1038/labinvest.3780212. [DOI] [PubMed] [Google Scholar]
- Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006;24:2412–2419. doi: 10.1634/stemcells.2006-0006. [DOI] [PubMed] [Google Scholar]
- Lokmic Z, Stillaert F, Morrison WA, Thompson EW, Mitchell GM. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J. 2007;21:511–522. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- Stillaert F, Findlay M, Palmer J, Idrizi R, Cheany S, Messina A, Abberton K, Morrison W, Thompson EW. Host rather than graft origin of Matrigel-induced adipose tissue in the murine tissue-engineering chamber. Tissue Eng. 2007;13:2291–2300. doi: 10.1089/ten.2006.0382. [DOI] [PubMed] [Google Scholar]