Summary
Expression of innate immune genes such as β-defensins is induced in airway epithelium by bacterial components via activation of NF-κB. We show here that live Gram-negative bacteria can similarly stimulate this pathway, resulting in upregulation of the β-defensin tracheal antimicrobial peptide (TAP) in primary cultures of bovine tracheal epithelial cells (TECs), by a Toll-like receptor 4 (TLR4)-mediated pathway. The Gram-negative airway pathogen Bordetella bronchiseptica possesses a type III secretion system previously suggested to inhibit the nuclear translocation of NF-κB in a cell line by immunohistochemistry. We therefore hypothesized that this pathogen might interfere in the innate immune response of the epithelium. Exposure of TECs to wild-type B. bronchiseptica suppressed the activation of NF-κB and the subsequent induction of TAP mRNA levels, whereas a type III secretion-defective strain did not. These results suggest a mechanism for bacterial evasion of the innate immune response in the airway, which could allow for the observed persistent colonization of this pathogen.
Introduction
Bacterial colonization of the respiratory tract is prevented in part by the presence of the innate immune response, which provides an initial line of defence against invading pathogens through physical and cellular mechanisms (Diamond et al., 2000a). One component of host response to infection of the airway is β-defensins, cationic peptides, 28–42 amino acids in length, with a broad spectrum of antimicrobial activity [reviewed in the study by Diamond and Bevins (1998)]. Tracheal antimicrobial peptide (TAP), the first of this family of antimicrobial peptides to be discovered, is a 38-amino acid peptide purified from bovine tracheal mucosa (Diamond et al., 1991), expressed in the ciliated epithelial cells of the airway (Diamond et al., 1993). TAP mRNA levels in cultured tracheal epithelial cells (TECs) are induced upon stimulation with heat-inactivated bacteria and LPS (Diamond et al., 1996). Furthermore, cytokines that are produced as part of a subsequent, localized inflammatory response, also induce TAP expression in the airway (Diamond et al., 2000b), as well as expression of its human orthologue hBD2 (Becker et al., 2000). The signalling pathway that is induced upon LPS stimulation activates the p50/p65 heterodimer of NF-κB, which is then able to bind to the NF-κB consensus sequence in the 5′ flanking region of the TAP (Diamond et al., 2000b) and hBD2 (O'Neil et al., 1999; Tsutsumi-Ishii and Nagaoka, 2002) genes, resulting in the upregulation of transcription.
Despite the defences provided by the innate immune response, some respiratory pathogens are able to establish infection in the respiratory tract. Bacteria in the Bordetella genus are aerobic, Gram-negative pathogens, often associated with respiratory disease in mammals. Virulence factors necessary for colonization of Bordetella bronchiseptica are regulated by the bvgAS locus (Cotter and Miller, 1994). One recently described group of virulence factors expressed during the bvg+ phase of B. bronchiseptica consists of the proteins associated with the type III secretion system (Yuk et al., 1998), a mechanism used by some Gram-negative bacteria to deliver proteins directly to the host cell cytosol and can modulate host cell functions (Galan and Collmer, 1999). For example, toxic factors secreted through the type III secretion mechanism associated with the intestinal pathogen Yersinia enterocolitica are able to inhibit MAP kinase pathways and NF-κB signalling pathways in the host cell (Orth et al., 1999), resulting in suppression of TNF-α production in Yersinia-infected macrophages (Beuscher et al., 1995; Ruckdeschel et al., 1997; Boland and Cornelis, 1998).
Many of the effectors of the bvgAS locus have yet to be characterized. One known component is the bscN locus, which, like the yscN locus of Yersinia, encodes an ATPase required for the secretion of virulence factors through the type III secretion system (Yuk et al., 1998). A mutant strain of B. bronchiseptica containing an in frame deletion of bscN (WD3) exhibits a decrease in the secretion of several unknown proteins and failure to maintain colonization of the trachea in a rat infection model when compared with the wild-type (RB50) strain (Yuk et al., 1998). This suggests that the type III secretion effector proteins are required for persistent colonization of the trachea. Furthermore, when the rat lung epithelial cell line L2 is stimulated with the wild-type RB50 strain, large aggregates of NF-κB are observed in the cytoplasm by immunofluorescence microscopy. However, cultures infected with the mutant WD3 strain exhibited normal NF-κB translocation to the nucleus after TNF-α stimulation (Yuk et al., 2000). This suggests that type III secreted product(s) of B. bronchiseptica may be able to inhibit NF-κB activation and the subsequent upregulation of innate immune genes.
We hypothesize that virulence factors associated with the type III secretion system of B. bronchiseptica, through sequestration of NF-κB, are able to inhibit downstream innate immune responses, such as the expression of defensins, enabling its colonization of the airway. While the initial study on the effect of the Bordetella type III system was performed on a lung epithelial cell line, B. bronchiseptica is specifically interacts with and colonizes the trachea. Therefore we examined the pathogen–host interaction of the RB50 and WD3 strains of B. bronchiseptica and primary cultures of bovine TECs. We report that the expression of TAP is suppressed in bovine TEC upon infection with the wild-type strain of B. bronchiseptica through inhibition of Toll-like receptor 4 (TLR4)-mediated NF-κB activation.
Results
As bacteria and bacterial components have been shown to induce responses in airway epithelial cells through different pathways, it was important to first determine the initial mechanism of recognition, and whether live bacteria stimulates the same pathway as the previously studied LPS. Primary cultures of bovine TECs express TLR2, 3, 4 and 9 mRNA as determined by reverse transcription polymerase chain reaction (RT-PCR) with human primers and by Northern blot analysis with human probes (data not shown). In order to determine if binding to TLR4 is necessary for the induction of TAP gene expression by live Gram-negative bacteria, we used Rhodobacter sphaeroides diphosphoryl lipid A (RsDPLA), an LPS antagonist at the level of TLR4 (Lien et al., 2000). Bovine TECs were preincubated with 10 μg ml−1 of RsDPLA for 1 h prior to stimulation with Pseudomonas aeruginosa LPS or two strains of live B. bronchiseptica (moi = 100 : 1) for 18 h. The results shown represent three independent experiments with similar results. Semi-quantitative RT-PCR analysis revealed that TAP mRNA was upregulated in response to P. aeruginosa LPS alone (Fig. 1), as was shown previously (Diamond et al., 1996). However, TAP expression was inhibited when cultures were incubated with RsDPLA prior to LPS stimulation. A weak upregulation was observed with RsDPLA alone, possibly because of the presence of bacterial lipoprotein in the RsDPLA preparation. Despite this, RsDPLA was still able to decrease TAP expression in response to LPS. In addition, we observed an increase in TAP mRNA levels in response to both strains of the live bacteria. This response was inhibited by preincubation with RsDPLA, suggesting that the predominant mechanism of recognition of live Gram-negative bacteria is through TLR4.
Fig. 1. Stimulation of TAP expression by LPS and bacteria via TLR4 in bovine TEC.
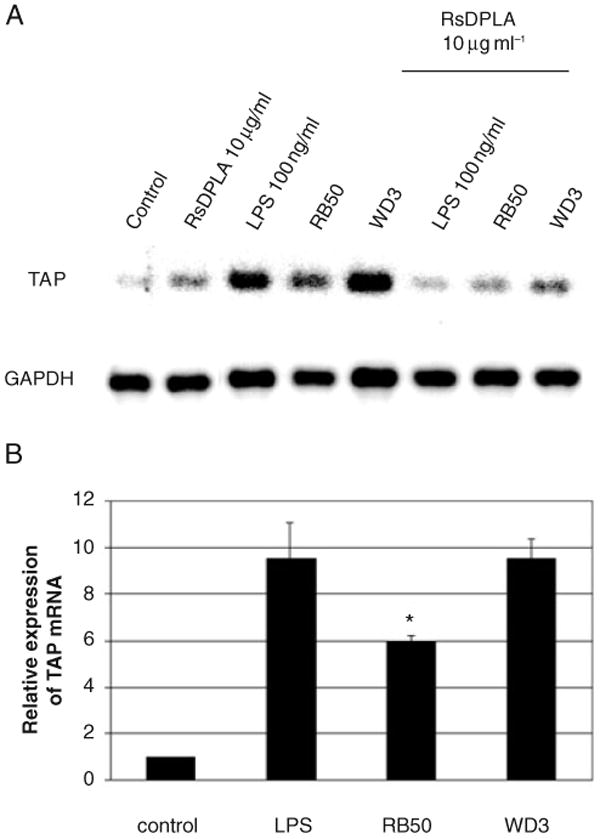
A. TECs were pretreated with 10 μg ml−1 RsDPLA for 1 h prior to stimulation with 100 ng ml−1 of P. aeruginosa LPS or live B. bronchiseptica for 18 h. Dose–response studies revealed that 10 μg ml−1 of RsDPLA was the lowest concentration necessary to observe the inhibition of TAP expression (not shown). Total RNA was subjected to semi-quantitative RT-PCR for 15 cycles, followed by Southern blot hybridization, as described in Experimental procedures.
B. Suppression of TAP upregulation by the type III secretion system of B. bronchiseptica. TEC were challenged with live B. bronchiseptica for 6 h. Graphical representation of the data (n = 3) indicates the mean of the fold increase over the untreated control samples ± standard error of the mean. Significance (*) of the difference between RB50 and WD3-stimulated cells was determined by t-test analysis, when P < 0.05.
To determine whether protein levels concomitantly increase, we performed mass spectrometry analysis on partially purified cellular extracts from unstimulated cells or cells stimulated with LPS. The results indicated a peak corresponding to TAP (m/z = 4083 Da) only in the stimulated cells (Fig. 2B), demonstrating that LPS induction of TAP gene expression results in increased protein production.
Fig. 2.
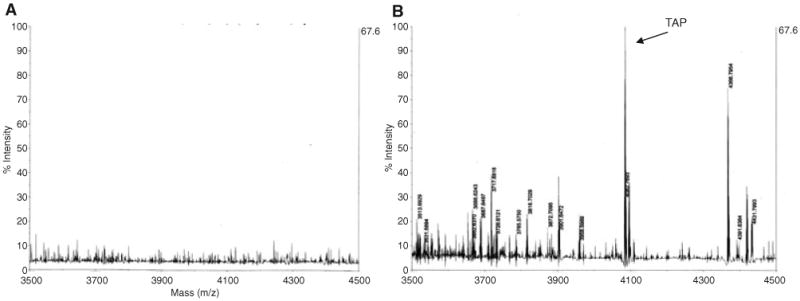
Detection of TAP peptide by mass spectrometry. Primary cultures of bovine TEC were incubated in the absence (A) or the presence (B) of 100 ng ml−1 Pseudomonas aeruginosa LPS for 18 h. Cells were lysed, and cytoplasmic extracts were partially purified by C-18 chromatography, and subjected to high resolution mass spectrometry as described in Experimental procedures.
Our previous results indicated that LPS stimulation of BTE resulted in activation and nuclear translocation of NF-κB (Diamond et al., 1996). Stimulation of BTE with TNF also results in upregulation (Russell et al., 1996), and examination by electrophoretic mobility shift assay (EMSA) indicates that this inflammatory cytokine also activates NF-κB in these cells (data not shown). To further examine the pathways leading to TAP expression, we utilized NF-κB SN50, a peptide that is able to inhibit translocation of the p50-containing NF-κB active complex to the nucleus. Semi-quantitative RT-PCR analysis revealed that cultures stimulated with LPS or recombinant human (rh) TNFα demonstrated an increase in TAP expression (Fig. 3A). Cultures pretreated with NF-κB SN50 prior to stimulation with 100 ng ml−1 P. aeruginosa LPS or rhTNF exhibited significant reduction in TAP mRNA levels (Fig. 3B). These results suggest that TAP mRNA expression in primary cultures of bovine TECs via both TLR4 and TNF is dependent on the translocation of NF-κB p50 subunit to the nucleus, and that interruption of this activation could lower TAP levels in the airway. When TECs were preincubated with SN50, followed by challenge with live B. bronchiseptica, we observed a 41.8% reduction in mRNA levels compared with cultures in the absence of SN50 (data not shown). This suggests that while stimulation of cells with purified LPS predominantly activates an NF-κB-mediated pathway, live bacteria stimulate several pathways, some of which activate the innate immune response through other transcription factors.
Fig. 3. Role of NF-κB activation in TAP upregulation.
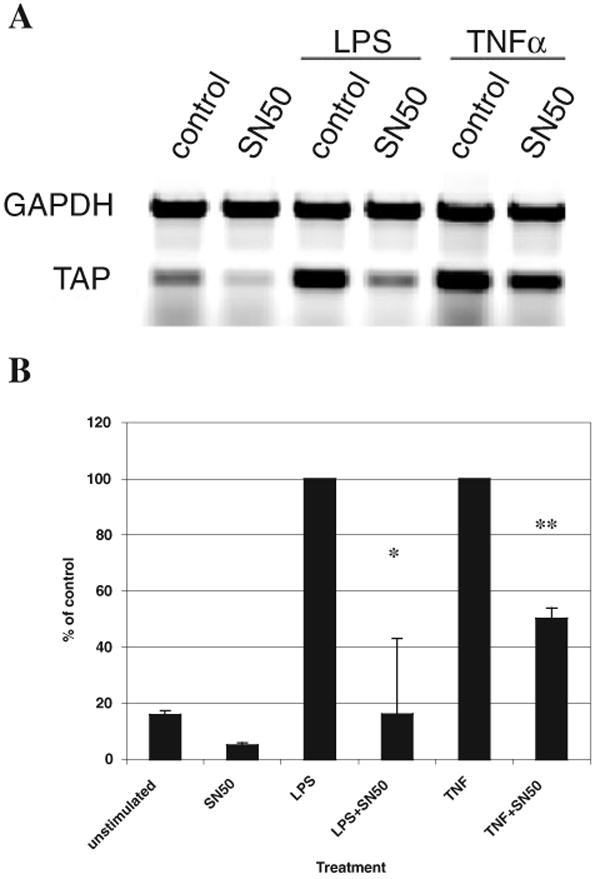
A. TECs were pretreated with 20 μM of SN50 for 1 h prior to stimulation with 100 ng ml−1 of P. aeruginosa LPS, 100 ng ml−1 or of rhTNFα for 18 h. Semi-quantitative RT-PCR was used to examine TAP and GAPDH expression. The products were visualized by gel electrophoresis and ethidium bromide staining. The blot shown is representative of three experiments.
B. Graphical representation of the data (n = 3) is presented as a percentage of the control treated with LPS or rhTNFα, which is set as 100%, ±standard error of the mean. Significance of the difference between cells treated with and without SN50 stimulated with LPS (*), or rhTNFα (**) was determined by t- test analysis, when P < 0.05. The optimal, sublethal concentration of SN50 was determined by dose–response studies (not shown).
A closer examination of the results shown in Fig. 1 showed a differential induction of TAP mRNA by the two different strains of B. bronchiseptica. As the growth kinetics of the two strains are the same, and TEC viability was the same after culture with both the mutant and wild-type strains (92% as measured by trypan blue staining), we further examined this response. Quantitation of the amplified TAP product revealed that there was a 10-fold increase in TAP expression in response to WD3, whereas there was only a sixfold increase in TAP expression in response to RB50 (Fig. 1B). This 40% reduction in the upregulation of TAP mRNA by RB50 is statistically significant as measured by t-test. As the WD3 strain of B. bronchiseptica is defective in its type III secretion mechanism, it can be inferred that the difference in TAP expression in response to the RB50 and the WD3 strain is attributed to a protein associated with the type III secretion machinery of B. bronchiseptica. Indeed, both strains, when heat-inactivated, show no difference in TAP activation, nor did conditioned-media from either culture, nor purified LPS from both strains (data not shown).
In order to examine the potential role of the bscN locus of B. bronchiseptica on activation of NF-κB binding activity in infected bovine TECs, we performed EMSA on nuclear extracts from bovine TEC cultures cocultured with each of the two strains. TECs were incubated with P. aeruginosa LPS or B. bronchiseptica strains RB50 or WD3 at an moi of 1000:1 for 1 h. Nuclear extracts were incubated with radioactively labelled, double-stranded oligonucleotide for the NF-κB consensus sequence of the TAP gene (nucleotides −165 to −197). We observed two complexes that were specific to the NF-κB consensus sequence (Fig. 4), subsequently determined by supershift assays (data not shown) to correspond to the p50/p50 homodimer (lower band) and the p50/p65 heterodimer (upper band). Cell cultures challenged with the RB50 strain had lower levels of NF-κB binding activity in the nucleus when compared with those challenged with the WD3 type III secretion-mutant strain. Using the same nuclear extracts, an EMSA was performed with labelled oligonucleotide to the NF-IL6 consensus binding sequence found in the 5′ flanking region of the TAP gene. No change in levels of protein binding was seen in nuclei from any sample [similar to what we previously observed with LPS (Diamond et al., 2000b)], and thus this acts as a control for protein concentrations in the nuclear extracts.
Fig. 4.
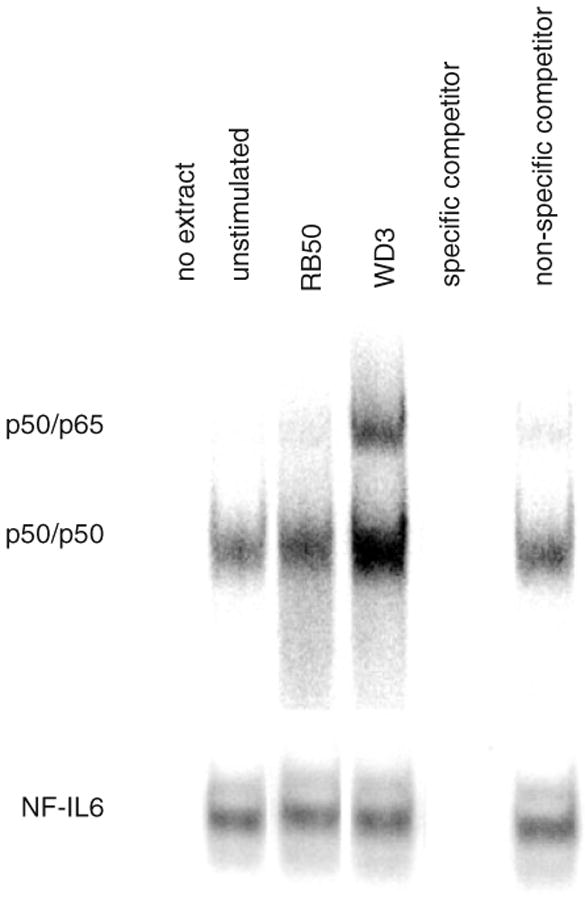
Inhibition of NF-κB binding by B. bronchiseptica. TECs were stimulated with viable RB50 or WD3 strains of B. bronchiseptica at an moi of 1000 : 1 for 1 h. Nuclear extracts were obtained, and 40 μg were incubated with [γ-32P]-ATP labelled, double-stranded oligonucleotide (ds-TAP/NF32) containing the NF-κB consensus sequence from the 5′ flanking region of the TAP gene (upper gel). Competition experiments were performed to determine specificity of binding by using unlabelled specific (ds-TAP/NF32) and non-specific (ds-TAP/NFmut32) double-stranded oligonucleotides. The blot shown is representative of two separate experiments. The same extracts were used in an EMSA with labelled oligonucleotide containing the NF-IL6 consensus sequence from the 5′ flanking region of the TAP gene (lower gel).
To identify the pathway inhibited by this factor, we examined steps known to be targeted by factors from other bacteria. Type III secretion factors of Yersinia inhibit the extracellular signal-regulated kinase (ERK) 1/2 MAP kinase pathway (Ruckdeschel et al., 1997). Stimulation of TEC with LPS induces the phosphorylation of ERK 1/2, and this is suppressed by the ERK phosphorylation inhibitor U0126 (Fig. 5A). Inhibition of ERK phosphorylation by U0126, however, does not lead to a suppression of LPS-stimulated TAP upregulation (Fig. 5B), even to the basal level of phosphorylation we routinely observe in unstimulated cells (lane 1). Furthermore, there was no observable difference between levels of phosphorylated ERK 1/2 in cells stimulated with RB50 compared with WD3 (Fig. 5C), indicating that the type III secretion factor is specific for a step in the pathway leading to NF-κB activation, and not to the Src-mediated pathway identified by Li et al. in the LPS-mediated induction of mucin gene expression in human TEC (Li et al., 1998). While the type III secretion system of B. bronchiseptica has been shown to induce ERK phosphorylation in bone marrow-derived dendritic cells (Skinner et al., 2004), LPS does not significantly induce ERK in dendritic cells [as confirmed in the study by Skinner et al. (2004)], but epithelial cells do respond to LPS with induction of ERK phosphorylation. Thus, irrespective of type III secretion status, the cells will respond to the bacteria with an increase in phosphorylation of ERK.
Fig. 5. Activation of the ERK pathway by LPS and live bacteria.
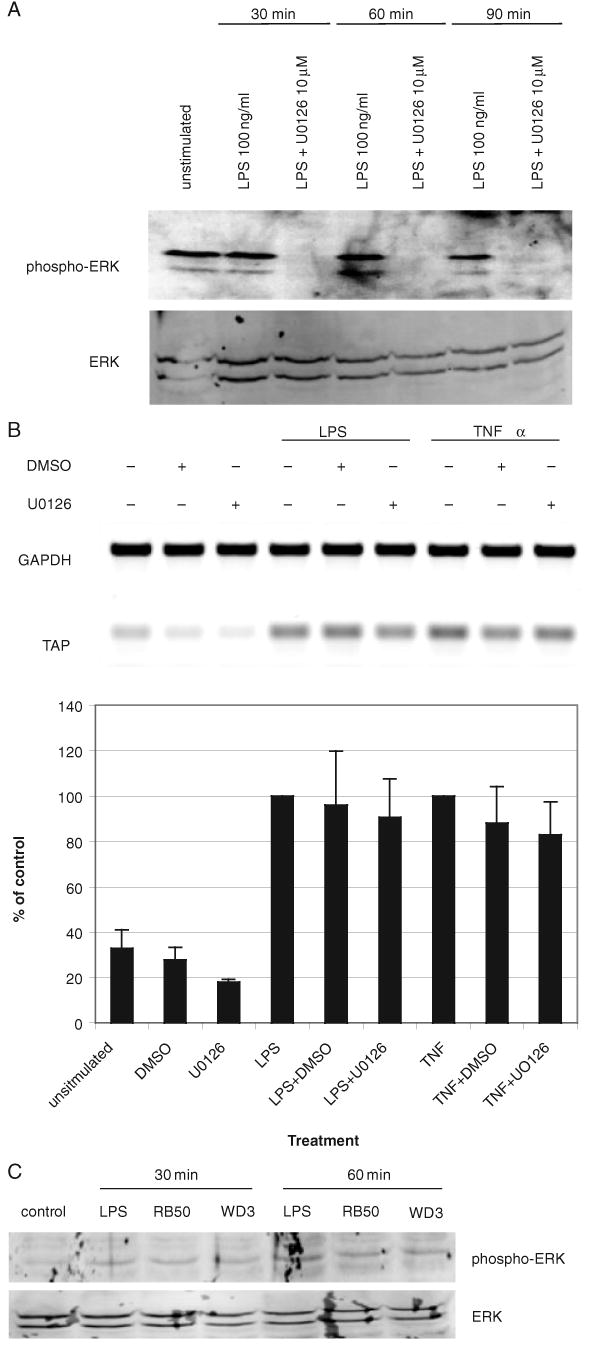
A. Inhibition of ERK phosphorylation of LPS-stimulated cells by U0126. TECs were stimulated with 100 ng ml−1 P. aeruginosa LPS for 30, 60 or 90 min, and ERK phosphorylation was determined by Western blot analysis. Phosphorylated ERK is shown in the upper panel, and total ERK is shown in the lower panel.
B. TAP upregulation is not dependent on the ERK pathway. TECs were pretreated with 10 μM of U0126 or DMSO as the vehicle control for 2 h prior to stimulation with 100 ng ml−1 of P. aeruginosa LPS, or 100 ng ml−1 of rhTNFα for 18 h. Semi-quantitative RT-PCR was used to examine TAP and GAPDH expression. The products were visualized by gel electrophoresis and ethidium bromide staining (upper panel). The blot shown is representative of three experiments. Graphical representation of the data (lower panel, n = 3) is presented as a percentage of the control treated with LPS, or rhTNFα alone, which is set as 100%, ±standard error of the mean.
C. The effect of B. bronchiseptica on ERK 1/2 phosphorylation. Total protein from TEC stimulated with 100 ng ml−1 P. aeruginosa LPS or the RB50 or WD3 strains of B. bronchiseptica (moi = 1000:1) for 30 or 60 min were subjected to Western blot analysis, as above.
It was also observed that the YopJ locus from Yersina inhibits the phosphorylation and degradation of IκB (Schesser et al., 1998). We found no difference in phosphorylated IκB levels in these cells by Western blot analysis (data not shown), suggesting that the type III secretion factor from B. bronchiseptica does not act on the same targets as those from Yersinia.
Infection of model human epithelia with non-pathogenic strains of Salmonella (S. typhimurium and S. pullorum) decreases IL-8 transcription by attenuating the ubiquitination and subsequent degradation of IκBα (Neish et al., 2000). TEC stimulated with either LPS or B. bronchiseptica showed no difference between levels of ubiquitinated IκBα (as measured by immunoprecipitation with an antibody to human IκBα) in cells stimulated with either strain of bacteria (data not shown).
Discussion
Bacterial pathogens require the ability to evade the innate host defence response in order to establish colonization in the respiratory tract. One recently described evasion mechanism involves the inhibition of NF-κB activation by type III secretion factors from Yersinia and Bordetella (Orth et al. 1999; 2000; Yuk et al., 2000). Inhibition of signalling pathways (Palmer et al., 1999) and cytokine production has been associated with type III secretion systems employed by other pathogenic bacteria as well (Beuscher et al., 1995; Ruckdeschel et al., 1997; Boland and Cornelis, 1998). Here, we report a significant reduction in TAP mRNA expression induced by the wild-type RB50 strain compared with the type III-deficient WD3 strain of B. bronchiseptica. Thus, the decrease in TAP expression in response to RB50 appears to be attributed to the virulence factors associated with the type III secretion system. While extent of suppression in our in vitro system (approximately 40% of the response to mutant bacteria) was not complete, this may be because of the experimental conditions, as with the inhibition of TNF activation by SN50 (to approximately 50% of uninhibited levels), or to the stimulation of NF-κB-independent pathways, as suggested by the 41.8% inhibition of bacterial induction by SN50.
The observed decrease in the binding activity of NF-κB in nuclear extracts from RB50-infected cells when compared with WD3-infected cells implies that the virulence factors associated with the type III secretion system inhibit NF-κB activation in the infected bovine TECs. While the specific molecular target of this inhibition remains to be elucidated, we have determined that it acts at a point in the pathway leading to NF-κB translocation, and not in an alternative LPS-mediated pathway identified by Li et al. (1998). Furthermore, it does not share a target with the type III secretion systems from either Yersinia or Salmonella, suggesting that it has evolved this evasion mechanism independently. As the response to live bacteria was reduced to unstimulated levels by RsDPLA, suggesting TLR4 was the primary pattern recognition receptor for this pathogen, we did not examine the effect of the type III secretion factor on the activation by other TLR ligands. We have observed the expression of other TLRs in these cells (data not shown), and purified flagellin from other bacteria can activate TAP expression (D. Legarda, A. Salzman and G. Diamond, unpubl. results), presumably through interaction with TLR5. As the predominant pathway stimulated by TLR ligation activates NF-κB through all of the same intermediates, we can speculate that initial (or simultaneous) interaction with live B. bronchiseptica could also reduce the response to these microbe-associated patterns as well. Thus, this work supports a mechanism for the observation that Bordetella infections are associated with other respiratory infectious agents (Jackson et al., 2000). Based on our results, we can speculate that Bordetella can downregulate the host's response to infection, which would then make the host susceptible to superinfection by other respiratory pathogens.
Experimental procedures
Reagents
Tissue culture growth media, U0126 and LPS from P. aeruginosa were obtained from Sigma Chemical, St. Louis, MO. Recombinant human TNF-α was purchased from R&D Systems, Minneapolis, MN. SN50 was purchased from Biomol Research Laboratories, Inc., Plymouth Meeting, PA. Activity of the inhibitors was tested by Western blot analysis. RsDPLA was a generous gift from Dr Nilofer Qureshi, University of Missouri – Kansas City School of Medicine.
Bovine TECs
Cells were obtained and cultured as previously described (Wu et al., 1985a,b; Diamond et al., 1996). Tissue obtained from bovine tracheas was washed in Dulbecco modified Eagle medium (DMEM) and was incubated in a 0.1% protease solution (Sigma Chemical) supplemented with 1% antibiotic/antimycotic (Gibco BRL, Grand Island, NY), and 1% Fungizone® (Gibco BRL) for 48 h at 4°C. Prior to agitation 10% foetal bovine serum (Gibco BRL) was added to the tissue. The cell suspension was filtered through Nitex filters (60 microns) (Sefaramerica, Depew, NY), centrifuged, and plated onto tissue culture dishes coated with a collagen gel (Vitrogen 100®, Collagen Biomedical, Palo Alto, CA). The cells were maintained in FDU media, which contained 48% DMEM, 48% F-12, 2% Ultroser G™ (Biosepra, Cergy-Saint-Christophe, France), 1% antibiotic/antimycotic (Gibco BRL), 0.5% Fungizone® (Gibco BRL) and 0.5% gentamicin (Gibco BRL), in a humidified incubator at 37°C and 5% CO2. Under these conditions, the cells are able to maintain epithelial characteristics, including the presence of active cilia. The cultures used for experimentation were from the first passage and were approximately 80–100% confluent.
Semi-quantitative RT-PCR
Approximately 1 × 106 primary bovine TECs were harvested after stimulation, and mRNA was extracted using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). For some experiments, on-column DNase digestion (Qiagen, Valencia, CA) was used in conjunction with the RNeasy Mini kit. One microgram of mRNA was reverse transcribed with 200 units of Superscript™ II RNase H− Reverse Transcriptase (Invitrogen Corporation), 40 units of RNase OUT™ Ribonuclease Inhibitor (Recombinant), 0.25 μg of Oligo(dT)12–18 Primer, 5 μM MgCl2, 4 mM of each dNTP, in 1×PCR buffer [20 mM Tris-HCl (pH 8.4), 50 mM KCl]. The reaction was performed at 42°C for 15 min, 99°C for 5 min and 5°C for 5 min. PCR conditions were optimized to obtain mid-logarithmic phase amplification. PCR was performed using the entire cDNA sample, with 12.5 units of Taq DNA Polymerase (Invitrogen Corporation), 1 mM MgCl2, 10× PCR buffer supplied by Invitrogen, which consisted of 200 mM Tris-HCl (pH 8.4) and 500 mM KCl, and oligonucleotide primers specific to the genes of interest, in a final volume of 100 μl. The reaction was performed using an initial denaturing step at 95°C for 3 min, followed by 25 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 2 min. An additional extension step of 72°C for 15 min followed the completed cycles. Semi-quantitative PCR analysis involved examining the PCR product in the mid-logarithmic phase of amplification as determined by titration. The RT-PCR products were electrophoresed on a 2% agarose gel stained with ethidium bromide, visualized by PhosphorImage or Typhoon 8600 Scanner (Molecular Dynamics, Sunnyvale, CA), and analysed by ImageQuant imaging software (Molecular Dynamics). Using this software, the product was quantitated by densitometry analysis and normalized to the GAPDH signal. All results represent three independent analyses unless otherwise stated. In some cases, we performed amplification for 15 cycles, followed by detection by Southern blotting with [γ-32P]-ATP-labelled TAP oligonucleotide and [α-32P]-CTP-labelled GAPDH cDNA using standard techniques.
The sequences of the bovine beta-defensin oligonucleotide primers are 5′-GCCAGCATGAGGCTCCAT-3′ (sense) and 5′-AACAGGTGCCAATCTGT-3′ (antisense) (Ryan et al., 1998). The sequences of the bovine GAPDH oligonucleotide primers are 5′-TGGCAAAGTGGACATCGTCG-3′ (sense) and 5′-TGGCGTGGACAGTGGTCATAAGTC-3′ (antisense). These oligonucleotides produced a 166 bp TAP product and a 467 bp GAPDH product.
Bordetella bronchiseptica
Growth conditions
Wild-type (RB50) and mutant (WD3) strains of B. bronchiseptica (Yuk et al., 1998; Cotter and Miller, 1994) were cultured on Bordet-Gengou agar (Difco, Detroit, MI) supplemented with 5% sheep blood (Quad Five, Ryegate, MT) and 1% glycerol, and liquid cultures were grown in Stainer–Scholte medium (Yuk et al., 1998; Cotter and Miller, 1997; Martinez de Tejada et al., 1996; Cotter and Miller, 1994), which contained 1% casamino acids, 63 mM l-glutamic acid sodium salt, 2 mM l-proline, 43 mM NaCl, 4% KH2PO4, 3 mM KCl, 492 μM MgCl2, 143 μM CaCl2 and 50 mM Tris base, supplemented with 330 μM l-cysteine, 36 μM FeSO4, 32 μM niacin, 228 μM glutathione and 2 mM ascorbic acid. In order to activate BvgAS and to maintain the bacterial strains in Bvg+-phase, the bacterial colonies and liquid cultures were grown to mid-log phase at 37°C, and liquid cultures were maintained at 37°C prior to cell culture infection. To obtain heat-inactivated cultures, bacteria were incubated at 65°C for 30 min. Conditioned media from the bacterial cultures was obtained by passing the liquid cultures through a 0.2 micron syringe filter.
Infection conditions
Bacterial infections of bovine TEC cultures were performed in six-well tissue culture plates. The TECs were washed two to three times in 1×PBS, prior to culture in antibiotic-free FDU for 12–16 h prior to bacterial infection. Bacterial strains were aliquoted according to the moi used in the experiment, centrifuged at approximately 2000 g for 2 min, washed twice and resuspended in prewarmed, antibiotic-free FDU. The bacterial suspensions were added to the cells, and subjected to centrifugation for 10 min at 1500 RPMI in a swinging-bucket rotor. The cell cultures were >90% viable after bacterial infection, as determined by trypan blue exclusion assay.
Nuclear and cytoplasmic extract preparation
Bovine TECs were grown to approximately 80–100% confluence in 10 cm tissue culture Petri dishes, harvested with trypsin-EDTA, and rinsed twice with cold PBS. Extracts were prepared according to a modified protocol from Clontech Laboratories. (Palo Alto, CA). Cells were resuspended in five times the cell pellet volume of lysis buffer (10 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 μM DTT, 1 mM PMSF), incubated for 15 min on ice, and then centrifuged for 5 min, 4°C, at 420 g. The supernatant was discarded and the pellet was resuspended in twice the cell pellet volume of lysis buffer. To lyse the cells, the cell suspension was drawn through a syringe with a 27-guage needle 10 times. The suspension was then centrifuged for 20 min, 4°C, at 11 000 g. The supernatant containing the cytoplasmic proteins was retained for further use. The nuclear pellet was resuspended in 2/3 the cell pellet volume of extraction buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 25% (v/v) glycerol, 0.01 M DTT, 10 mM PMSF). The nuclei were disrupted with a mechanical dounce homogenizer (Kontes Glass Company, Vineland, NJ). The nuclear suspension was disrupted at 4°C for 30 min and then centrifuged at 20 000 g for 5 min. The nuclear extract was then transferred to a chilled microcentrifuge tube, and its concentration was determined by Bio-Rad Protein Assay (Bio-Rad Laboratories).
Electrophoretic mobility shift assay
Electrophoretic mobility shift assays were performed as previously described (Diamond et al., 2000b). Nuclear extract (40 μg) was incubated with poly dl-dC (Sigma Chemical) and 10× binding buffer (100 mM Tris pH 7.5, 0.5 M NaCl, 1 mM EDTA, 10 mM DTT, 40% glycerol, 100 μg ml−1 salmon sperm DNA) for 15 min at room temperature prior to the addition of labelled, double-stranded probes. Samples were then incubated for 30 min at 30°C and separated by gel electrophoresis on a 6% non-denaturing polyacrylamide gel in 0.25× Tris-borate EDTA. The oligonucleotides were labelled with [γ-32P]-ATP using T4 polynucleotide kinase (Invitrogen Corporation), and complementary oligonucleotides were annealed at 65°C for 2 min to create the double-stranded probes. The sequence of the sense NF-κB oligonucleotide (TAP/NF32) is 5′-AGCTTTTTCTGGGGTTTTCCCCAGCCTCAT-3′. The sense sequence of the oligonucleotide used for competition experiments is 5′-AGCTTTTTCTCTCATTTTCCCCAGCCTCAT-3′. This is a mutant form of the NF-κB oligonucleotide (TAP/NFmut32). The NF-IL6 oligonucleotide is 5′-AGCTTTTTCTGGGGTTTTCCCCAGCCTCAT-3′; the mutant competitor is 5′-AGCTTTTTCTCTCATTTTCCCCAGCCTCAT-3′.
Mass spectrometry analysis
Cytoplasmic extracts were obtained from bovine TEC that had been incubated with or without 100 ng ml−1 LPS for 18 h, as described above. The protein extracts were concentrated through a C-18 Ziptip (Millipore, Billerica, MA) and subsequently analysed by MALDI-TOF mass spectrometry by the Center for Advanced Proteomics, UMDNJ-NJMS.
Statistics
t-test was used to calculate the P-values, and differences were considered significant at P < 0.05.
Acknowledgments
We thank Dr Nilofer Qureshi (University of Missouri – Kansas City School of Medicine) for RsDPLA, the Molecular Resource Facility of UMDNJ (Dr Robert Donnelly, director) for oligonucleotide synthesis and DNA sequencing, the UMDNJ Mass Spectrometry facility (Dr Hong Li, director) for MALDI-TOF analysis, and Danielle Laube for technical assistance and helpful discussions. This work was supported by US Public Health Service grants R01 DE14897 and R01 HL67871 (G.D.) and R01 AI049346 (M.H.Y.), and by a STAR fellowship from the US EPA (to M.K.P.).
References
- Becker MN, Diamond G, Verghese MW, Randell SH. CD14-dependent lipopolysaccharide-induced beta-defensin-2 expression in human tracheobronchial epithelium. J Biol Chem. 2000;275:29731–29736. doi: 10.1074/jbc.M000184200. [DOI] [PubMed] [Google Scholar]
- Beuscher HU, Rodel F, Forsberg A, Rollinghoff M. Bacterial evasion of host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland A, Cornelis GR. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Miller JF. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol Microbiol. 1997;24:671–685. doi: 10.1046/j.1365-2958.1997.3821741.x. [DOI] [PubMed] [Google Scholar]
- Diamond G, Bevins CL. Beta-defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunop. 1998;88:221–225. doi: 10.1006/clin.1998.4587. [DOI] [PubMed] [Google Scholar]
- Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a novel cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Jones DE, Bevins CL. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc Natl Acad Sci USA. 1993;90:4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Russell JP, Bevins CL. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000a;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- Diamond G, Kaiser V, Rhodes J, Russell JP, Bevins CL. Transcriptional regulation of β-defensin gene expression in tracheal epithelial cells. Infect Immun. 2000b;68:113–119. doi: 10.1128/iai.68.1.113-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- Jackson LA, Cherry JD, Wang SP, Grayston JT. Frequency of serological evidence of Bordetella infections and mixed infections with other respiratory pathogens in university students with cough illnesses. Clin Infect Dis. 2000;31:3–6. doi: 10.1086/313911. [DOI] [PubMed] [Google Scholar]
- Li JD, Feng W, Gallup M, Kim JH, Gum J, Kim Y, Basbaum C. Activation of NF-kB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc Natl Acad Sci USA. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de Tejada G, Miller JF, Cotter PA. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol Microbiol. 1996;22:895–908. doi: 10.1046/j.1365-2958.1996.01538.x. [DOI] [PubMed] [Google Scholar]
- Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, et al. Prokaryotic regulation of epithelial responses by inhibition of ikappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- O'Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- Orth K, Palmer LE, Bao ZQ, Stewart S, Rudolph AE, Bliska JB, Dixon JE. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science. 1999;285:1920–1923. doi: 10.1126/science.285.5435.1920. [DOI] [PubMed] [Google Scholar]
- Orth K, Xu Z, Mudgett MB, Bao ZQ, Palmer LE, Bliska JB, et al. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science. 2000;290:1594–1597. doi: 10.1126/science.290.5496.1594. [DOI] [PubMed] [Google Scholar]
- Palmer LE, Pancetti AR, Greenberg S, Bliska JB. YopJ of Yersinia spp. is sufficient to cause downregulation of multiple mitogen-activated protein kinases in eukaryotic cells. Infect Immun. 1999;67:708–716. doi: 10.1128/iai.67.2.708-716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel K, Machold J, Roggenkamp A, Schubert S, Pierre J, Zumbihl R, et al. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. Correlation with its inhibitory effect on tumor necrosis factor-alpha production. J Biol Chem. 1997;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- Russell JP, Diamond G, Tarver AP, Scanlin TF, Bevins CL. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect Immun. 1996;64:1565–1568. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan LK, Rhodes J, Bhat M, Diamond G. Expression of beta-defensin genes in bovine alveolar macrophages. Infect Immun. 1998;66:878–881. doi: 10.1128/iai.66.2.878-881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schesser K, Spiik AK, Dukuzumuremyi JM, Neurath MF, Pettersson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- Skinner JA, Reissinger A, Shen H, Yuk MH. Bordetella type III secretion and adenylate cyclase toxin synergize to drive dendritic cells into a semimature state. J Immunol. 2004;173:1934–1940. doi: 10.4049/jimmunol.173.3.1934. [DOI] [PubMed] [Google Scholar]
- Tsutsumi-Ishii Y, Nagaoka I. NF-kappaB-mediated transcriptional regulation of human beta-defensin-2 gene following lipopolysaccharide stimulation. J Leukoc Biol. 2002;71:154–162. [PubMed] [Google Scholar]
- Wu R, Nolan E, Turner C. Expression of tracheal differentiated functions in serum-free hormone-supplemented medium. J Cell Physiol. 1985a;125:167–181. doi: 10.1002/jcp.1041250202. [DOI] [PubMed] [Google Scholar]
- Wu R, Yankaskas J, Cheng E, Knowles MR, Boucher R. Growth and differentiation of human nasal epithelial cells in culture. Am Rev Respir Dis. 1985b;132:311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]
- Yuk MH, Harvill ET, Miller JF. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]
- Yuk MH, Harvill ET, Cotter PA, Miller JF. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol Microbiol. 2000;35:991–1004. doi: 10.1046/j.1365-2958.2000.01785.x. [DOI] [PubMed] [Google Scholar]


