Abstract
Background
Several antioxidant nutrients have been reported to be inversely associated with asthma. A study was undertaken to assess the independent associations of these nutrients with asthma in adults.
Methods
A nested case‐control study was performed in 515 adults with physician diagnosed asthma and 515 matched controls using dietary data obtained from 7 day food diaries. The main outcome measures were physician diagnosed asthma and current symptomatic asthma (diagnosed asthma and self‐reported wheeze within the previous 12 months).
Results
Cases were similar to controls in age, sex, social class, and daily energy intake but had a lower median intake of fruit (132.1 v 149.1 g/day, p⩽0.05). 51.5% of the population reported zero consumption of citrus fruit; relative to these individuals, people who consumed >46.3 g/day had a reduced risk of diagnosed and symptomatic asthma (OR adjusted for potential confounders 0.59 (95% CI 0.43 to 0.82) and 0.51 (95% CI 0.33 to 0.79), respectively). In nutrient analysis, dietary vitamin C and manganese were inversely and independently associated with symptomatic asthma (adjusted OR per quintile increase 0.88 (95% CI 0.77 to 1.00) for vitamin C and 0.85 (95% CI 0.74 to 0.98) for manganese), but only manganese was independently associated with diagnosed asthma (OR 0.86 (95% CI 0.77 to 0.95)). Adjusted plasma levels of vitamin C were significantly lower in symptomatic cases than in controls (54.3 v 58.2 μmol/l, p = 0.003).
Conclusions
Symptomatic asthma in adults is associated with a low dietary intake of fruit, the antioxidant nutrients vitamin C and manganese, and low plasma vitamin C levels. These findings suggest that diet may be a potentially modifiable risk factor for the development of asthma.
Keywords: antioxidants, asthma, diet, epidemiology
The increase in the prevalence of asthma in developed countries1,2,3,4,5 and the remarkable differences in asthma prevalence between countries suggests that environmental exposures are important in the development of this disease.6,7 It has been hypothesised that dietary changes associated with a westernised lifestyle may have contributed to the increase in asthma in the developed world.8,9 Epidemiological evidence of an inverse association of dietary fruit intake with pulmonary function10,11 and respiratory symptoms12,13 suggests that dietary antioxidants may modify the development of respiratory disease in susceptible individuals. This is supported by reports of an inverse association between specific antioxidant nutrients including vitamin C,14,15 vitamin E,15,16,17 β‐carotene,16,18 zinc,14,19 manganese,19 copper,14 selenium20,21,22 and asthma. However, similar associations have been reported for nutrients not known to have antioxidant activity such as magnesium,19,23 calcium,17 niacin,14,19 and fatty acids.15,24
It seems unlikely that each of these nutrients is independently associated with the risk of asthma. The identification of so many associations could be related to a failure of some studies to adequately adjust for the effects of nutrients that are correlated with the nutrient of interest, making it unclear whether the reported associations are independent.25 An alternative explanation is that, rather than any specific nutrient being protective, asthma may be associated with a generally less healthy diet that is part of a less healthy lifestyle. For example, Hijazi et al identified a significant inverse association between dietary calcium and asthma in Saudi children, but this was not significant after adjustment for lifestyle exposures including place of residence and maternal education.17
We have conducted a nested case‐control study of the association between diet and asthma in the Norfolk arm of the European Prospective Investigation of Cancer (EPIC‐Norfolk). Using comprehensive dietary data, we have assessed the association between specific foods and nutrients and the presence of asthma in adults. Our objective was to determine whether the apparent association between dietary antioxidants and asthma can be attributed to a “healthy diet” and lifestyle, or to the protective effect of specific foods or nutrients.
Methods
The EPIC‐Norfolk cohort is a population based cohort of men and women aged 45–75 years recruited from 35 general practices in and around the city of Norwich, UK, the primary objective of which is to prospectively assess the association between diet and the development of chronic disease. Detailed descriptions of the operation and characteristics of the EPIC‐Norfolk cohort26 and the collection of dietary data27,28 are available elsewhere. In brief, between 1993 and 1998 volunteers completed a health and lifestyle questionnaire (HLQ) and attended for a health check at which anthropometric measurements were made, spirometric tests performed, and non‐fasting plasma vitamin C levels measured.28 The HLQ provided information on smoking history, education, occupation, and usual level of physical activity.
Potential cases were first identified by a positive response to the question: “Has your doctor ever told you that you have asthma?” in the HLQ. Current smokers were excluded. Ex‐smokers were included only if they had stopped smoking before the age of 50 and at least 20 years before the study. People with a history of diabetes, myocardial infarction, or cancer were excluded as they may have altered their diet. Controls were identified by a negative response to the question: “Has your doctor ever told you that you have asthma?” and the absence of airflow obstruction (FEV1/FVC >0.75) on spirometric testing. The same smoking and co‐morbidity exclusion criteria were used for cases and controls. Each case was matched to a control by sex, age (±3 years), date of health check (±3 months) and general practitioner. All potential cases and controls were sent an additional questionnaire, the Norfolk respiratory health survey (NRHS),29 in which they recorded respiratory symptoms experienced in the previous 12 months. Since asthma may develop at any stage in life and regress in later life, we used this questionnaire to identify cases with current symptomatic asthma (self‐reported wheeze within the previous 12 months). In the analysis we therefore used two case definitions—physician diagnosed asthma and current symptomatic asthma (physician diagnosed asthma with self‐reported wheeze in the previous 12 months).
Dietary data for this analysis were obtained from 7 day food diaries. At the health check a trained interviewer instructed participants on how to complete the diary. A 24 hour recall formed the first day; the remaining 6 days were completed prospectively at home. Intake of individual foods and nutrients excluding dietary supplements was calculated using the DINER programme.27 This instrument has been validated against biomarkers of nutrient intake.30,31 The mean (SD) interval between completion of the food diary and posting of the NRHS was 3.4 (1.2) years. A sample size of 550 cases and 550 controls was derived based on 90% power and 95% confidence to detect a 50% difference in risk between high and low levels of nutrient consumption (that is, odds ratio (OR) of 1.5). Approval for the study was given by the local ethics committee.
Statistical analysis
The association between diet and asthma was assessed using specific foods, individual nutrients, and plasma vitamin C levels. Analyses were first performed between all cases and controls and then between symptomatic cases (self‐reported wheeze within the previous 12 months) and their matched controls. Nutrients included in the analysis were selected on the basis of previous studies reporting an association with asthma and the availability of complete data from the DINER programme. The nutrients assessed were vitamins C and E, niacin, riboflavin, carotene, calcium, copper, selenium, magnesium, manganese, and zinc. Fruit and vegetables are an important source of many of these nutrients and also an important source of folate. We made the a priori decision to include folate in the analysis although there have been no previous reports of an association with asthma. Continuous variables were compared between matched cases and controls using paired Student's t test or Wilcoxon rank sum for paired data and t test or Kruskal‐Wallace test for unpaired data. Proportions were compared using a χ2 or McNemar test as appropriate. Conditional logistic regression models were used to test for a linear trend across quintiles of increasing intake of each food or nutrient and the risk of diagnosed asthma and of symptomatic asthma.
More than 20% of the population reported zero consumption of citrus fruit, soft summer fruit, apples and salad vegetables, so it was not practical to use quintiles of consumption for these foods. For these foods individuals who reported zero intake were classified as “non‐consumers” and formed the reference group, while the remainder of the population were divided by the median intake into moderate consumers (consumption below the median level of intake) and high consumers (consumption above the median level of intake). Lifestyle exposures including social class, pack years smoked, body mass index (BMI), usual level of physical activity (inactive, moderately inactive, moderately active, and active),32 and education level (below GCE, GCE level, Advanced level and degree level) that may confound the association between diet and asthma were adjusted for by inclusion as covariates in the regression model. Foods or nutrients that were significant in these analyses (p⩽0.05) were included in multiple regression analysis to determine whether they made an independent contribution after mutual adjustment. All analyses were performed using Stata.
Results
By 1998, 20 076 people had completed the baseline HLQ; 1614 responded positively to the question on doctor diagnosed asthma, 974 of whom met the smoking and other inclusion criteria for the study. Five hundred and seventy two of these individuals completed the NRHS and were successfully matched to controls. Complete dietary data from food diaries were available for 515 matched case‐control pairs (table 1). A higher proportion of controls reported smoking >1 pack year than cases. Cases were more likely to report taking vitamin C containing supplements but the difference was not statistically significant. Although there was no difference in energy intake between cases and controls, controls had a greater daily intake of fruit (table 1), with a significant trend for a decreased risk of diagnosed asthma with increasing fruit intake (adjusted OR per quintile increase in intake 0.86 (95% CI 0.78 to 0.95), p = 0.002). A comparison of high (upper quintile) versus low (bottom quintile) intake showed a protective effect for high consumption of fruit (adjusted OR 0.46 (95% CI 0.30 to 0.70), p<0.001) and vegetables (adjusted OR 0.64 (95% CI 0.42 to 0.97), p = 0.04). However, after mutual adjustment, only dietary fruit (adjusted OR 0.87 (95% CI 0.77 to 0.98), p = 0.003) was significantly associated with a reduced risk of diagnosed asthma.
Table 1 Characteristics and consumption of food groups by cases and controls.
| Cases (n = 515) | Controls (n = 515) | |
|---|---|---|
| Men, % (n) | 32.6 (168) | 32.6 (168) |
| Mean (SD) age (years) | 58.5 (8.7) | 58.4 (8.6) |
| Mean (SD) BMI (kg/m2) | 26.2 (3.8) | 25.9 (3.7) |
| Ex‐smokers, % (n) | 24.1 (124)‡ | 29.1 (150)‡ |
| Pack years* | 7.0 (11.0) | 9.0 (15.4) |
| Mean (SD) FEV1 (l) | 2.12 (0.69)‡ | 2.62 (0.61)‡ |
| Social class, % (n) | ||
| I | 8.9 (45) | 6.9 (35) |
| II | 41 (208) | 37.9 (191) |
| III | 34.5 (175) | 41.1 (207) |
| IV | 11.1 (56) | 10.9 (55) |
| V | 4.5 (23) | 3.2 (16) |
| Vitamin C supplements, % (n) | 14.0 (72) | 10.9 (56) |
| Median (IQR) fruit (g/day) | 132.1 (145.2)† | 149.1 (138.5)† |
| Median (IQR) vegetables (g/day) | 96.9 (81.7) | 96.6 (81.8) |
| Median (IQR) cereals (g/day) | 30.0 (45.0) | 28.1 (46.6) |
| Median (IQR) fish (g/day) | 30.4 (38.3) | 28.6 (30.5) |
| Median (IQR) dairy products (g/day) | 249.8 (196.4) | 265.9 (207.7) |
| Median (IQR) meat (g/day) | 115.8 (88.1) | 107.2 (72.4) |
| Median (IQR) pulses/beans/ lentils (g/day) | 22.4 (33.4) | 21.8 (36.8) |
| Mean (SD) total energy (kJ) | 7895 (2158) | 7904 (1987) |
Figures are mean (SD), median (IQR), or percentage (number) as indicated.
BMI, body mass index; FEV1, forced expiratory volume in 1 second; ex‐smokers, people who reported ever smoking >1 pack year of cigarettes; vitamin C supplements, self‐reported taking of vitamin supplements know to contain vitamin C in the food diary.
*Total pack years smoked by ex‐smokers.
†p<0.05, ‡p⩽0.001, difference between cases and controls.
Data from the NRHS identified 187 (36.3%) individuals who had not experienced wheeze in the preceding 12 months. These asymptomatic cases were not significantly different in age, sex, age at diagnosis, or smoking exposure from the symptomatic cases (table 2). However, far fewer of the asymptomatic individuals reported current use of asthma medication and they had significantly better pulmonary function than symptomatic cases, suggesting that the absence of symptoms was more likely explained by a remission in their asthma than by better treatment. Restriction of the analysis to the 328 pairs in which the case was symptomatic showed a trend for reduced risk of symptomatic asthma with increasing fruit consumption (adjusted OR per quintile increase in intake 0.86 (95% CI 0.76 to 0.96), p = 0.009) but not vegetable consumption.
Table 2 Characteristics of symptomatic and asymptomatic cases.
| Symptomatic cases (n = 328) | Asymptomatic cases (n = 187) | |
|---|---|---|
| Men, % (n ) | 34.8 (112) | 28.9 (54) |
| Mean (SD) age (years) | 58.4 (8.9) | 58.5 (8.3) |
| Mean (SD) BMI (kg/m2) | 26.3 (3.9) | 25.9 (3.6) |
| Ex‐smokers, % (n) | 25.0 (82) | 22.6 (42) |
| Mean (SD) age diagnosed (years) | 32.9 (20.8) | 29.4 (21.7) |
| Asthma treatment, % (n) | 84.2 (276)‡ | 44.4 (83)‡ |
| Mean (SD) FEV1 (l) | 2.06 (0.72)† | 2.21 (0.63)† |
Figures are mean (SD) or percentage (number) as stated.
Symptomatic cases are those who reported wheeze within the previous 12 months.
BMI, body mass index; FEV1, forced expiratory volume in 1 second; ex‐smokers, individuals who reported previously smoking >1 pack year; age diagnosed, reported age at which asthma started; asthma treatment, self‐reported current use of medication for asthma.
†p⩽0.02, ‡p⩽0.001, difference between symptomatic and asymptomatic cases.
The association between dietary fruit and vegetables was assessed in more detail using subgroups of fruit and vegetables: citrus fruits (oranges, grapefruits, lemons), summer fruits (berries), apples and “other fruit” (all remaining fruits), leafy vegetables (cabbage) and salad vegetables. Of these foods only “other fruit” and leafy vegetables could reliably be categorised into quintiles of consumption (⩽20% reported zero intake). There was a significant trend for a reduced risk of diagnosed asthma with increasing consumption of “other fruit” (adjusted OR per quintile increase in intake 0.90 (95% CI 0.82 to 0.98), p = 0.02). Zero consumption of citrus fruit, soft summer fruit, apples, and salad vegetables was reported by 51.5%, 77.9%, 30.6%, and 25.5% of the population, respectively. The association between dietary intake of these foods with diagnosed asthma and symptomatic asthma is shown in table 3. Relative to non‐consumption, a high consumption of apples and citrus fruit was associated with a reduced risk of diagnosed asthma. After mutual adjustment, a high intake of citrus fruit remained significantly associated with a reduced risk of diagnosed asthma (adjusted OR 0.64 (95% CI 0.46 to 0.89), p = 0.008); a high intake of apples and other fruit were no longer significant (adjusted OR 0.75 (95% CI 0.53 to 1.07) and 0.66 (95% CI 0.44 to 1.00), respectively). Moreover, when the analysis was restricted to symptomatic cases, only citrus fruit was associated with a reduced risk of asthma, with consumption of ⩾45.8 g/day being associated with a 50% reduction in risk (table 3).
Table 3 Odds ratio for the presence of symptomatic asthma associated with moderate and high consumption of specific types of fruit and vegetables.
| Type of fruit or vegetables | All cases | Symptomatic cases | ||||
|---|---|---|---|---|---|---|
| Adjusted* OR (95% CI) | Adjusted* OR (95% CI) | |||||
| Moderate consumption | High consumption | p (trend) | Moderate consumption | High consumption | p (trend) | |
| Citrus fruit | 0.88 (0.65 to 1.20) | 0.59‡ (0.43 to 0.82) | 0.002 | 0.75 (0.51 to 1.11) | 0.51‡ (0.33 to 0.79) | 0.002 |
| Summer fruit | 1.15 (0 .77 to 1.70) | 1.12 (0.73 to 1.72) | 0.50 | 1.03 (0.63 to 1.69) | 1.48 (0.83 to 2.63) | 0.24 |
| Apples | 0.78 (0.58 to 1.05) | 0.68† (0.49 to 0.96) | 0.03 | 0.77 (0.52 to1.12) | 0.71 (0.46 to 1.09) | 0.11 |
| Salad vegetables | 0.85 (0.61 to 1.18) | 0.88 (0.62 to 1.24) | 0.52 | 0.73 (0.48 to1.09) | 0.73 (0.48 to 1.11) | 0.16 |
OR (CI), odds ratio and 95% confidence interval for the presence of diagnosed asthma or symptomatic asthma relative to non‐consumers of each food.
Consumption categories: moderate consumption was defined as consumption below the median level of consumption in consumers and high consumption as above the median level in consumers. Range of intake: citrus fruits 0.7–46.2 g/day (moderate) and ⩾46.3 g/day (high); summer fruit 0.4–18.5 g/day (moderate) and ⩾18.6 g/day (high); apples 2.0–48.0 g/day (moderate) and ⩾48.1 g/day (high); salad vegetables 0.3–20.5 g/day (moderate) and ⩾20.6 g/day (high).
*Matched analysis adjusted for pack years smoked, social class, BMI, increasing level of physical activity, and level of education.
†p⩽0.05, ‡p⩽0.002.
Nutrients
There was no difference in total calorie intake between cases and controls (table 1), or in the intake by cases and controls of total fat (71.7 (25.6) v 71.1 (22.4) g/day), saturated fat (23.8 (10.5) v 23.7 (9.0) g/day), or polyunsaturated fat (11.0 (4.8) v 11.1 (4.47) g/day). Table 4 shows the association of dietary nutrients with diagnosed and symptomatic asthma. Increasing intake of vitamin C, folate, calcium, and manganese were individually associated with a reduced risk of diagnosed asthma. However, after mutual adjustment, only the association with dietary manganese remained significant (adjusted OR per quintile increase 0.86 (95% CI 0.77 to 0.95), p = 0.002). Restriction of the analysis to symptomatic cases identified an inverse association between increasing dietary intake of vitamin C, folate, riboflavin, calcium, copper, and manganese. After mutual adjustment, only the association between vitamin C and manganese with symptomatic asthma remained significant (adjusted OR per quintile increase 0.88 (95% CI 0.77 to 1.00), p⩽0.05 for vitamin C and 0.85 (95% CI 0.74 to 0.98), p = 0.003 for manganese).
Table 4 Daily intake of nutrients in cases and controls and odds ratio (OR) for symptomatic asthma per quintiles increase in consumption.
| Nutrient | Median (IQR) daily intake | Adjusted*OR (CI) per quintile increase in intake | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | All cases | p value | Symptomatic cases | p value | |
| Vitamin C (mg) | 78.4 (56.0) | 85.0 (56.4) | 0.89 (0.81 to 0.97) | 0.007 | 0.84 (0.75 to 0.94) | 0.003 |
| Vitamin E (mg) | 6.7 (3.9) | 6.7 (3.3) | 0.96 (0.87 to 1.05) | 0.39 | 0.89 (0.79 to 1.00) | 0.51 |
| Folate (μg) | 250.0 (102.7)† | 263.3 (100.1)† | 0.89 (0 .81 to 0.98) | 0.01 | 0.84 (0.74 to 0.94) | 0.004 |
| Niacin (mg) | 1.8 (1.6) | 1.8 (1.6) | 1.07 (0.97 to 1.18) | 0.17 | 1.06 (0.94 to 1.20) | 0.35 |
| Riboflavin (mg) | 1.7 (0.7) | 1.7 (0.8) | 0.93 (0.84 to 1.01) | 0.10 | 0.87 (0.77 to 0.98) | 0.03 |
| Carotene (mg) | 1.8 (1.6) | 1.8 (1.6) | 0.95 (0.87 to 1.04) | 0.26 | 0.89 (0.79 to 1.00) | 0.05 |
| Calcium (mg) | 776.0 (326.8) | 825.4 (333.0) | 0.88 (0.80 to 0.97) | 0.01 | 0.84 (0.74 to 0.95) | 0.006 |
| Copper (mg) | 1.0 (0.4) | 1.1 (0.4) | 0.93 (0.84 to 1.02) | 0.12 | 0.88 (0.77 to 0.99) | 0.04 |
| Selenium (μg) | 51.7 (26.5) | 51.3 (25.4) | 0.99 (0.89 to 1.09) | 0.72 | 1.02 (0.90 to 1.15) | 0.76 |
| Magnesium (mg) | 275.1 (93.4) | 281.4 (97.4) | 0.94 (0.85 to 1.04) | 0.22 | 0.89 (0.78 to 1.01) | 0.07 |
| Manganese (mg) | 3.4 (1.6)‡ | 3.6 (1.4)‡ | 0.83 (0.76 to 0.91) | ⩽0.001 | 0.83 (0.73 to 0.93) | 0.002 |
| Zinc (mg) | 7.8 (3.0) | 7.8 (2.7) | 1.00 (0.91 to 1.11) | 0.94 | 0.98 (0.86 to 1.12) | 0.78 |
OR (CI), OR and 95% confidence interval for diagnosed and symptomatic asthma per quartile increase in consumption of each nutrient (linear model).
*Matched analysis adjusted for pack years smoked, social class, BMI, increasing level of physical activity and level of education.
†p⩽0.05, ‡p<0.001, difference in daily intake between cases and controls.
To avoid recruiting cases with smoking related chronic obstructive pulmonary disease (COPD), we excluded individuals who had smoked within 20 years of the study. However, a significant proportion of the cases and controls were ex‐smokers; exclusion of these individuals left 335 case‐control pairs in whom the adjusted odds ratios for diagnosed asthma were 0.40 (95% CI 0.23 to 0.70, p⩽0.001) for top versus bottom quintiles of fruit intake, and 0.61 (95% CI 0.41 to 0.90, p = 0.01) for high versus zero intake of citrus fruit, with a significant trend for reduced risk with increasing intake of vitamin C and manganese (OR per quintile increase in intake 0.90 (95% CI 0.81 to 1.00), p⩽0.05 and 0.84 (95% CI 0.76 to 0.94), p = 0.003, respectively). COPD is more prevalent in an elderly population. We therefore repeated the analysis after exclusion of all individuals age 55 years or older. In the remaining 195 matched pairs a high intake of fruit and citrus fruit remained significantly associated with a reduced risk of diagnosed asthma (OR 0.41 (95% CI 0.19 to 0.88), p = 0.02 top versus bottom quintile of fruit, and OR 0.53 (95% CI 0.31 to 0.92), p = 0.02, high versus zero intake of citrus fruit). The association between vitamin C and manganese did not reach statistical significance (OR per quintile increase in intake 0.91 (95% CI 0.77 to 1.06) and 0.90 (95% CI 0.77 to 1.05) respectively), possibly because of the small sample size.
Plasma vitamin C
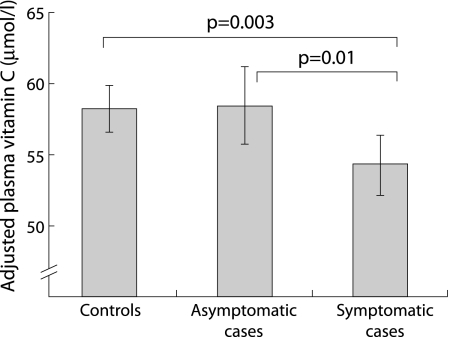
Plasma levels of vitamin C were available for 461 cases and 454 controls. Mean vitamin C levels were not significantly different between all cases (56.0 (19.00) μmol/l) and controls (58.1 (18.2) μmol/l). However, levels were significantly lower in symptomatic cases than either asymptomatic cases or controls, even after adjustment for potential confounding exposures (fig 1), with a 1 μmol/l increase in plasma vitamin C associated with a reduced risk of symptomatic asthma (adjusted OR 0.99 (95% CI 0.98 to 1.00), p = 0.04).
Figure 1 Mean plasma vitamin C levels in 454 controls, 167 asymptomatic cases with diagnosed asthma, and 294 symptomatic cases with diagnosed asthma adjusted for age, sex, pack years smoked, social class, body mass index, increasing level of physical activity, and level of education.
Discussion
In this population based study we found asthma in adults to be associated with a low dietary intake of fruit. We considered whether the apparent association between diet and asthma could be explained by a generally less healthy diet that in turn may be part of a less healthy lifestyle. However, we found no difference in total energy or fat intake between cases and controls. Moreover, the cases and controls were well matched for key demographic variables and the observed associations were independent of previous smoking history, BMI, social class, education level, and usual level of physical activity. The protective effect of fruit appeared to be related to the antioxidant nutrient vitamin C and was independent of the other nutrients investigated. We also found an inverse association between manganese and asthma. Manganese is found in a variety of foods, particularly grains and cereals,33 a high intake of which has been associated with a reduced risk of wheeze in childhood34 and of COPD in adults.35 Moreover, dietary manganese has been reported to be inversely associated with bronchial hyperreactivity19 and asthma in adults,22 although the association with asthma was lost after adjustment for other nutrients. In our study the higher intake of manganese in controls was not explained by differences in dietary cereal and grain. However, subsequent analysis has shown that median (IQR) daily intake of tea (another important dietary source of manganese) was significantly greater in controls than in cases (749.4 (602) g v 638.9 (270.3) g, p<0.001). Manganese has antioxidant properties36 and dietary intake of manganese may modify the activity of manganese dependent superoxide dismutase.37 The role of this enzyme in protecting the lung from oxidative damage has recently been reviewed38 and reduced levels of superoxide dismutase activity have been reported in both the lung39 and blood monocytes of people with asthma.40
Approximately one third of individuals with diagnosed asthma reported no wheeze within the previous 12 months, less than 50% of whom reported taking regular medication for asthma. Some of these individuals may have been misdiagnosed with asthma or their asthma may be in remission. The inclusion of these individuals as cases may have weakened the association between diet and asthma. Restriction of the analysis to symptomatic cases reduced the number of case‐control pairs by more than one third (from 515 to 328); however, the associations of citrus fruit, dietary vitamin C and manganese with asthma were maintained with a trend towards a greater protective effect—that is, the odds ratios became more extreme (see table 3 and 4). Three controls reported taking medications used in the treatment of asthma (without a diagnosis of asthma) and 58 reported wheeze in the NRHS. These 61 individuals may have undiagnosed asthma and their inclusion as controls may have attenuated the association between diet and asthma. However, we repeated the analysis after the exclusion of these individuals and the key associations remained unchanged.
A suggested weakness of previous studies is that they have focused on foods and nutrients in isolation.22 A particular strength of our study is the comprehensive nature of the dietary data. This enabled us to show that the association between vitamin C and manganese with asthma was independent of other nutrients. A potential limitation of food diary data is that the results may not be readily translated into accessible public health recommendations. We found that individuals in the highest quintile of fruit consumption had a reduced risk of asthma. Using data from a semi‐quantitative food frequency questionnaire which formed part of the HLQ, we found that all individuals in the top quintile reported eating fresh fruit at least 2–4 times with 90% eating fresh fruit every day. Consumption of at least one portion of a fresh fruit a day would therefore appear to be a reasonable public health recommendation.
Our observations are consistent with previous reports of an inverse association between dietary fruit10,12 and dietary vitamin C14,19 and respiratory symptoms. However, a recent population based study found no association between dietary vitamin C—as assessed by a food frequency questionnaire (FFQ)—and asthma.22 Although the population in that study was younger and had a higher proportion of smokers than our study, the negative findings of this and other studies10,16,41 may relate to methodological differences in recording dietary intake. Semi‐quantitative FFQs, which are widely used, may give less precise estimates for some foods and nutrients than the diary method used in our study. For example, Shaheen et al reported a significant inverse association between apple consumption and asthma but no association with citrus fruit.22 The authors used a 7 day weighted food diary in a small proportion of their study population to calibrate the FFQ data used for the main analysis. They found that, although apple consumption showed reasonable agreement, citrus fruit consumption showed poor agreement between the FFQ and the more detailed 7 day diary (online supplementary data).22 There was also similarly poor correlation for intake of vitamin C and manganese. The finding that FFQs may give imprecise estimates of vitamin C intake is further supported by studies that have reported an association between plasma vitamin C levels and wheeze but not with dietary vitamin C assessed by FFQ.14,15 Although it could be argued that low plasma levels in asthma reflect an increased utilisation of vitamin C, our results suggest this is unlikely to be the sole explanation as symptomatic asthma was associated with a significant reduction in dietary intake.
We have considered to what extent the results reported here could have resulted from chance variation, bias, or confounding exposures. The associations between fresh fruit, dietary vitamin C, dietary manganese, plasma vitamin C levels and asthma were highly significant and therefore unlikely to be explained by chance variation. However, as we looked at a number of dietary variables and performed multiple analyses, the possibility of a type I statistical error has to be considered. It is unlikely that our results are explained by reporting bias as the primary objective of the EPIC cohort is to assess the association between diet and cancer. Thus, at the time of recording their diet, neither cases nor controls would have been aware that the data would be used to assess the association between diet and asthma. Although our study was not prospective in design, we assessed the association between current symptomatic asthma and diet as recorded some 3 years before the study, making recall bias unlikely. Moreover, the association with dietary vitamin C was confirmed by plasma levels of vitamin C.
It has been suggested that dietary antioxidants could modify the manifestation of symptoms in adults susceptible to asthma.19 The results of our study are consistent with this hypothesis. We found that adults previously diagnosed with asthma who reported no wheeze had significantly higher plasma vitamin C levels than symptomatic individuals. This was associated with a higher median (IQR) dietary intake of vitamin C in asymptomatic than in symptomatic case (89.2 (54.4) v 76.8 (56.3) mg/day); although this did not reach statistical significance, the study was not powered to detect differences between symptomatic and asymptomatic cases. Contrary to this hypothesis, the Nurses Health Study found no association between dietary vitamin C and incident asthma. However, an effect of low vitamin C in this study may have been obscured by the generally high intake of vitamin C in these health professionals.16 A meta‐analysis of trials of vitamin C supplementation in the treatment of asthma did not find conclusive evidence of a benefit.42 Trials have been criticised for using pharmacological doses of vitamin C for short periods as a treatment for asthma;43 however, a recent trial of 16 weeks vitamin C supplementation showed no benefit.44 The fact that these studies have been inconclusive does not exclude the possibility that a habitual diet with an adequate amount of vitamin C may protect against the development of symptomatic asthma.
There are some limitations of our study. As this is an observational study we cannot exclude the possibility that cases may have changed their diet because of their asthma. However, in the HLQ, only 22 cases (4.3%) reported a change in diet because of allergy within the past 12 months. This does not exclude the possibility that some cases may have made longer term changes to their diet as a result of asthma. The EPIC cohort is middle aged and we cannot exclude the possibility that some of the cases may have had smoking related COPD. We think this is unlikely as only 25% of cases had ever smoked and the exclusion of these individuals did not affect the key associations with dietary citrus fruit, vitamin C, and manganese. Cases and controls in this study were selected from the same cohort, had similar key demographic characteristics, and we adjusted for additional potential confounding lifestyle exposures. However, we cannot completely exclude the possibility that the observed relationship is the result of residual confounding or the result of some unmeasured confounding exposure. As with previous studies, we did not include nutrient intake from supplements.22 Although we found no difference in the use of vitamin C containing supplements between cases and controls, taking supplements may in itself be a marker of a healthy lifestyle. However, subsequent analysis after the exclusion of supplement takers did not affect the association with citrus fruit, vitamin C, and manganese (data not shown). We excluded some nutrients because of incomplete food composition data (for example, n‐3 polyunsaturated fatty acids). We were unable to look prospectively at the association between diet and incident asthma. This would have required the NRHS to be posted serially to the EPIC‐Norfolk cohort which would have been beyond the scope of this study. Moreover, incident cases of asthma are uncommon in this age group. In the Nurses Health Study, for example, the incidence of asthma was only 1.17 cases per 1000 person years of follow up.16
In summary, we have found symptomatic asthma in adults to be associated with a low intake of the dietary antioxidants vitamin C and manganese. The low intake of vitamin C appears to be primarily associated with a diet deficient in fruit. Further work is required to determine the dietary differences resulting in a low intake of manganese. These findings may be of public health importance in understanding the apparent increase in the prevalence of asthma.
Abbreviations
BMI - body mass index
FEV1 - forced expiratory volume in 1 second
FVC - forced vital capacity
HLQ - health and lifestyle questionnaire
NRHS - Norfolk respiratory health survey
Footnotes
Bipen Patel is funded by the NHS Executive Anglia and Oxford R&D. Nicholas Wareham is a Wellcome Trust Senior Clinical Fellow. EPIC‐Norfolk is supported by grant funding from the Cancer Research Campaign, the Medical Research Council, the Stroke Association, the British Heart Foundation, the Department of Health, the Europe Against Cancer Programme Commission of the European Union, and the Ministry of Agriculture, Fisheries and Food.
The authors have no competing interests.
References
- 1.Anderson H R, Butland B K, Strachan D P. Trends in prevalence and severity of childhood asthma. BMJ 19943081600–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ninan T K, Russell G. Respiratory symptoms and atopy in Aberdeen schoolchildren: evidence from two surveys 25 years apart. BMJ 1992304873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haahtela T, Lindholm H, Bjorksten F.et al Prevalence of asthma in Finnish young men. BMJ 1990301266–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burney P G, Chinn S, Rona R J. Has the prevalence of asthma increased in children? Evidence from the national study of health and growth 1973–86. BMJ 19903001306–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peat J K, van den Berg R H, Green W F.et al Changing prevalence of asthma in Australian children. BMJ 19943081591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 19983511225–1232. [PubMed] [Google Scholar]
- 7.Anon Variations in the prevalence of respiratory symptoms, self‐reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur Respir J 19969687–695. [DOI] [PubMed] [Google Scholar]
- 8.Seaton A, Godden D J, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax 199449171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolcock A J. Asthma—disease of a modern lifestyle. Med J Aust 1996165358–359. [PubMed] [Google Scholar]
- 10.Cook D G, Carey I M, Whincup P H.et al Effect of fresh fruit consumption on lung function and wheeze in children. Thorax 199752628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strachan D P, Cox B D, Erzinclioglu S W.et al Ventilatory function and winter fresh fruit consumption in a random sample of British adults. Thorax 199146624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forastiere F, Pistelli R, Sestini P.et al Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. SIDRIA Collaborative Group, Italy (Italian Studies on Respiratory Disorders in Children and the Environment). Thorax 200055283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miedema I, Feskens E J, Heederik D.et al Dietary determinants of long‐term incidence of chronic nonspecific lung diseases. The Zutphen Study. Am J Epidemiol 199313837–45. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz J, Weiss S T. Dietary factors and their relation to respiratory symptoms. The Second National Health and Nutrition Examination Survey. Am J Epidemiol 199013267–76. [DOI] [PubMed] [Google Scholar]
- 15.Bodner C, Godden D, Brown K.et al Antioxidant intake and adult‐onset wheeze: a case‐control study. Aberdeen WHEASE Study Group. Eur Respir J 19991322–30. [DOI] [PubMed] [Google Scholar]
- 16.Troisi R J, Willett W C, Weiss S T.et al A prospective study of diet and adult‐onset asthma. Am J Respir Crit Care Med 19951511401–1408. [DOI] [PubMed] [Google Scholar]
- 17.Hijazi N, Abalkhail B, Seaton A. Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax 200055775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rautalahti M, Virtamo J, Haukka J.et al The effect of alpha‐tocopherol and beta‐carotene supplementation on COPD symptoms. Am J Respir Crit Care Med 19971561447–1452. [DOI] [PubMed] [Google Scholar]
- 19.Soutar A, Seaton A, Brown K. Bronchial reactivity and dietary antioxidants. Thorax 199752166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flatt A, Pearce N, Thomson C D.et al Reduced selenium in asthmatic subjects in New Zealand. Thorax 19904595–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone J, Hinks L J, Beasley R.et al Reduced selenium status of patients with asthma. Clin Sci 198977495–500. [DOI] [PubMed] [Google Scholar]
- 22.Shaheen S O, Sterne J A, Thompson R L.et al Dietary antioxidants and asthma in adults: population‐based case‐control study. Am J Respir Crit Care Med 20011641823–1828. [DOI] [PubMed] [Google Scholar]
- 23.Britton J, Pavord I, Richards K.et al Dietary magnesium, lung function, wheezing, and airway hyperreactivity in a random adult population sample. Lancet 1994344357–362. [DOI] [PubMed] [Google Scholar]
- 24.Hodge L, Salome C M, Peat J K.et al Consumption of oily fish and childhood asthma risk. Med J Aust 1996164137–140. [DOI] [PubMed] [Google Scholar]
- 25.Fogarty A, Britton J. The role of diet in the aetiology of asthma. Clin Exp Allergy 200030615–627. [DOI] [PubMed] [Google Scholar]
- 26.Day N, Oakes S, Luben R.et al EPIC‐Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 199980(Suppl 1)95–103. [PubMed] [Google Scholar]
- 27.Welch A A, McTaggart A, Mulligan A A.et al DINER (Data Into Nutrients for Epidemiological Research)—a new data‐entry program for nutritional analysis in the EPIC‐Norfolk cohort and the 7‐day diary method. Public Health Nutr 200141253–1265. [DOI] [PubMed] [Google Scholar]
- 28.Bingham S A, Welch A A, McTaggart A.et al Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public Health Nutr 20014847–858. [DOI] [PubMed] [Google Scholar]
- 29.Jarvis D, Lai E, Luczynska C.et al Prevalence of asthma and asthma‐like symptoms in young adults living in three east Anglian towns. Br J Gen Pract 199444493–497. [PMC free article] [PubMed] [Google Scholar]
- 30.McKeown N M, Day N E, Welch A A.et al Use of biological markers to validate self‐reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. Am J Clin Nutr 200174188–196. [DOI] [PubMed] [Google Scholar]
- 31.Day N, McKeown N, Wong M.et al Epidemiological assessment of diet: a comparison of a 7‐day diary with a food frequency questionnaire using urinary markers of nitrogen, potassium and sodium. Int J Epidemiol 200130309–317. [DOI] [PubMed] [Google Scholar]
- 32.Wareham N J, Jakes R W, Rennie K L.et al Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 20036407–413. [DOI] [PubMed] [Google Scholar]
- 33.Pennington J A, Young B E, Wilson D B.et al Mineral content of foods and total diets: the Selected Minerals in Foods Survey, 1982 to 1984. J Am Diet Assoc 198686876–891. [PubMed] [Google Scholar]
- 34.Ellwood P, Asher M I, Bjorksten B.et al Diet and asthma, allergic rhinoconjunctivitis and atopic eczema symptom prevalence: an ecological analysis of the International Study of Asthma and Allergies in Childhood (ISAAC) data. ISAAC Phase One Study Group. Eur Respir J 200117436–443. [DOI] [PubMed] [Google Scholar]
- 35.Tabak C, Smit H A, Heederik D.et al Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy 200131747–755. [DOI] [PubMed] [Google Scholar]
- 36.Chen M T, Sheu J Y, Lin T H. Protective effects of manganese against lipid peroxidation. J Toxicol Environ Health A 200061569–577. [DOI] [PubMed] [Google Scholar]
- 37.Davis C D, Greger J L. Longitudinal changes of manganese‐dependent superoxide dismutase and other indexes of manganese and iron status in women. Am J Clin Nutr 199255747–752. [DOI] [PubMed] [Google Scholar]
- 38.Kinnula V L, Crapo J D. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 20031671600–1619. [DOI] [PubMed] [Google Scholar]
- 39.Comhair S A, Bhathena P R, Dweik R A.et al Rapid loss of superoxide dismutase activity during antigen‐induced asthmatic response. Lancet 2000355624. [DOI] [PubMed] [Google Scholar]
- 40.Vachier I, Damon M, Le Doucen C.et al Increased oxygen species generation in blood monocytes of asthmatic patients. Am Rev Respir Dis 19921461161–1166. [DOI] [PubMed] [Google Scholar]
- 41.Grievink L, Smit H A, Ocke M C.et al Dietary intake of antioxidant (pro)‐vitamins, respiratory symptoms and pulmonary function: the MORGEN study. Thorax 199853166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur B, Rowe B H, Ram F S. Vitamin C supplementation for asthma. Cochrane Database Syst Rev 2001(4)CD000993. [DOI] [PubMed]
- 43.Hatch G. Vitamin C and asthma. In: Packer L, Fuchs J, eds. Vitamin C in health and disease. New York: Marcel Dekker, 1997279–294.
- 44.Fogarty A, Lewis S A, Scrivener S L.et al Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo‐controlled trial. Clin Exp Allergy 2003331355–1359. [DOI] [PubMed] [Google Scholar]