Abstract
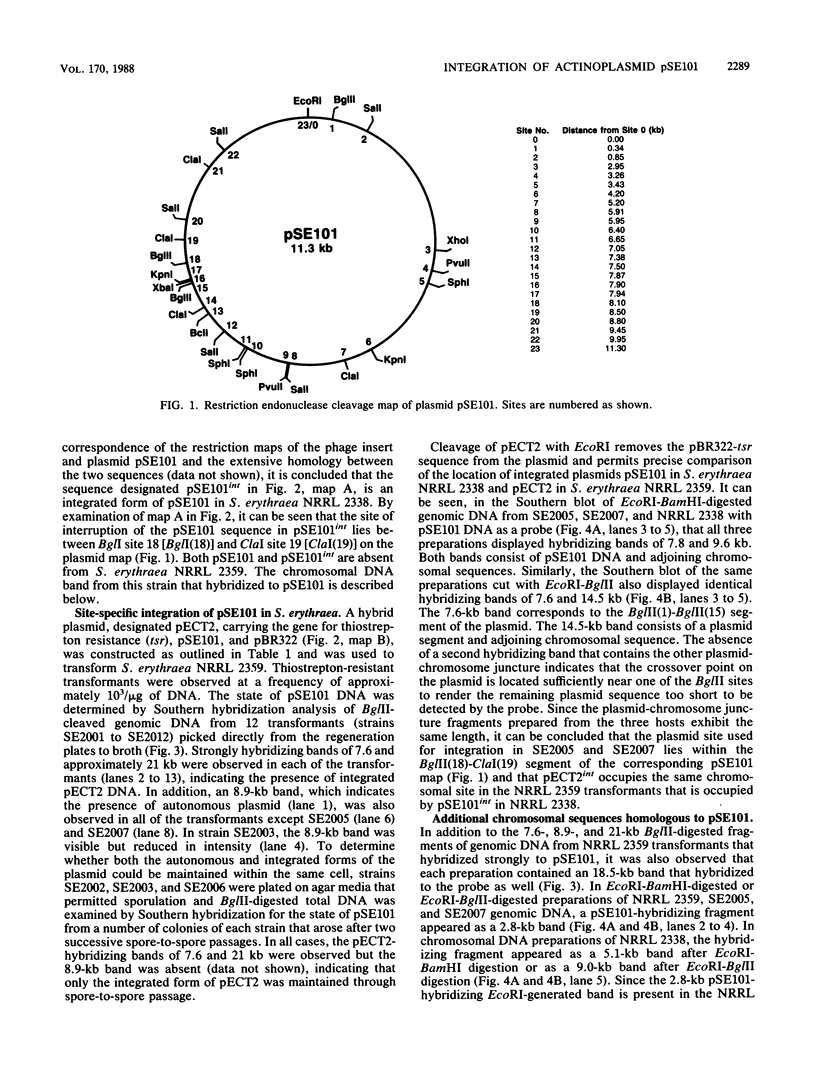
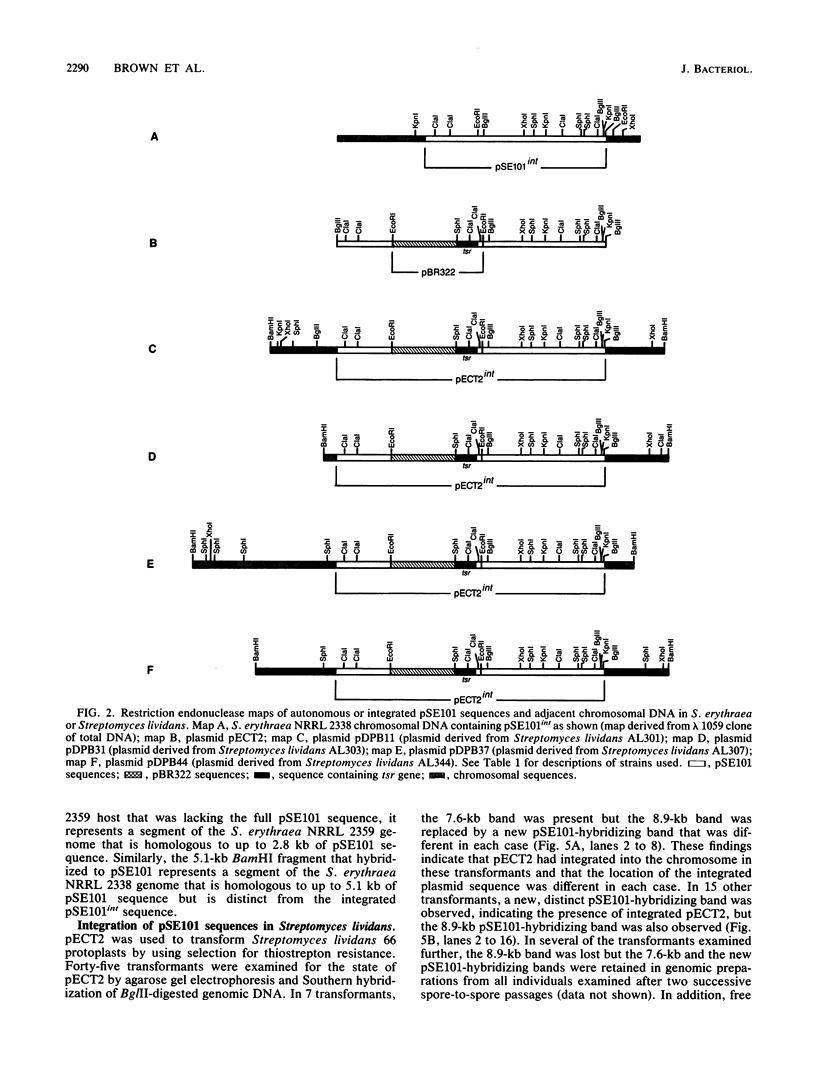
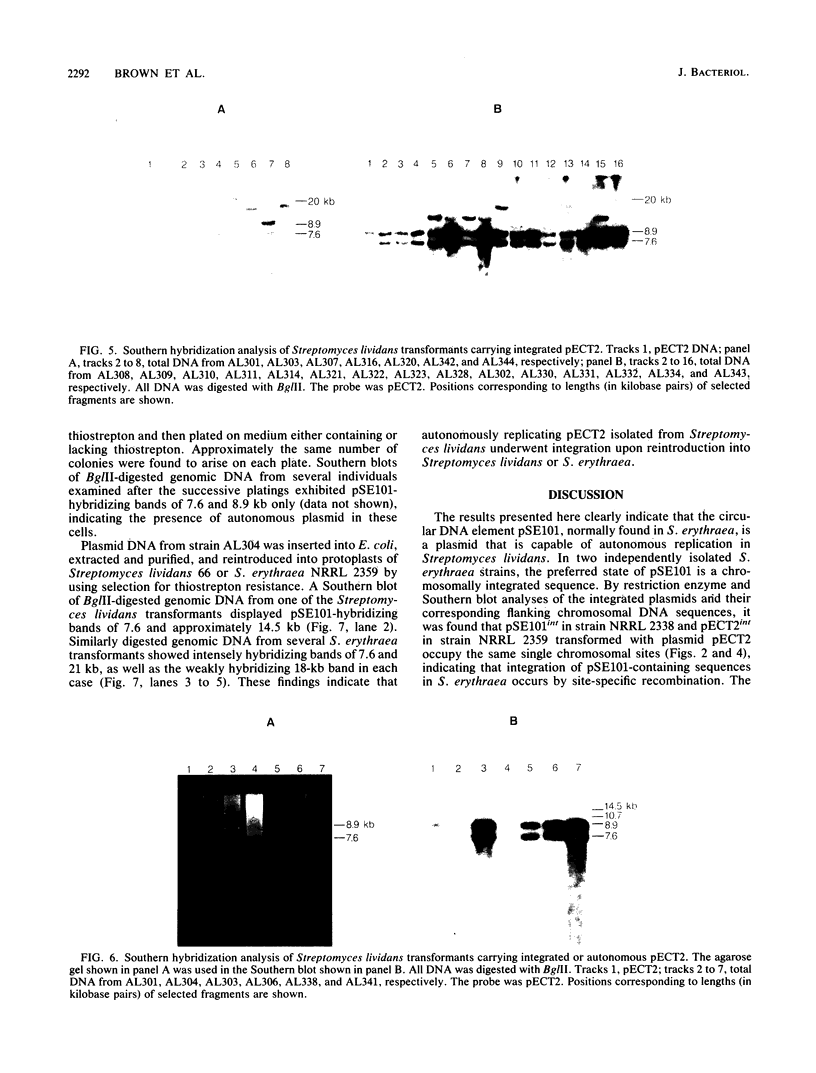
An 11.3-kilobase-pair plasmid, designated pSE101, exists in Saccharopolyspora erythraea NRRL 2338 as an integrated sequence (pSE101int) at a unique chromosomal location and in the free form in less than an average of 1 copy per 10 chromosomes. The plasmid sequence is missing from S. erythraea NRRL 2359. Restriction maps of the free and integrated forms of pSE101 showed point-to-point correspondence. Plasmid pECT2 was constructed by ligation of pSE101, pBR322, and the gene for thiostrepton resistance (tsr). When introduced by polyethylene glycol-mediated transformation into protoplasts of S. erythraea NRRL 2359, all thiostrepton-resistant regenerants examined were found to carry a single copy of pECT2 in the integrated state at a single chromosomal site. The chromosomal site of pECT2 integration in strain NRRL 2359 (attB) corresponded to the chromosomal location of pSE101int in strain NRRL 2338. The plasmid crossover site (attP) was mapped to the plasmid site that corresponded to the site of interruption of the plasmid sequence in the host carrying pSE101int, indicating that site-specific integrative recombination had occurred. An additional 2.8-kilobase-pair chromosomal sequence homologous to a segment of pSE101 was also observed in strains NRRL 2338 and NRRL 2359. After introduction of pECT2 into Streptomyces lividans, approximately half of the transformants examined were found to carry the plasmid as a stable, autonomously replicating element. The other half carried a single copy of pECT2 as an integrated sequence, but the location of pECT2int in Streptomyces lividans varied from one transformant to another. In each case, integrative crossover used the attP site. A model is proposed to account for the determination of the particular state of pSE101 in Streptomyces lividans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Ward J. M., Kieser T., Cohen S. N., Hopwood D. A. Excision of chromosomal DNA sequences from Streptomyces coelicolor forms a novel family of plasmids detectable in Streptomyces lividans. Mol Gen Genet. 1981;184(2):230–240. doi: 10.1007/BF00272910. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cohen A., Bar-Nir D., Goedeke M. E., Parag Y. The integrated and free states of Streptomyces griseus plasmid pSG1. Plasmid. 1985 Jan;13(1):41–50. doi: 10.1016/0147-619x(85)90054-x. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Hintermann G., Kieser T., Wright H. M. Integrated DNA sequences in three streptomycetes form related autonomous plasmids after transfer to Streptomyces lividans. Plasmid. 1984 Jan;11(1):1–16. doi: 10.1016/0147-619x(84)90002-7. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Jaoua S., Guespin-Michel J. F., Breton A. M. Mode of insertion of the broad-host-range plasmid RP4 and its derivatives into the chromosome of Myxococcus xanthus. Plasmid. 1987 Sep;18(2):111–119. doi: 10.1016/0147-619x(87)90038-2. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Klaus S., Krügel H., Walter F. Inverted repeats in the DNA of Streptomyces plasmids pMG110 and pMG120. Mol Gen Genet. 1984;197(1):143–149. doi: 10.1007/BF00327935. [DOI] [PubMed] [Google Scholar]
- Lydiate D. J., Ikeda H., Hopwood D. A. A 2.6 kb DNA sequence of Streptomyces coelicolor A3(2) which functions as a transposable element. Mol Gen Genet. 1986 Apr;203(1):79–88. doi: 10.1007/BF00330387. [DOI] [PubMed] [Google Scholar]
- Madon J., Moretti P., Hütter R. Site-specific integration and excision of pMEA100 in Nocardia mediterranei. Mol Gen Genet. 1987 Sep;209(2):257–264. doi: 10.1007/BF00329651. [DOI] [PubMed] [Google Scholar]
- Moretti P., Hintermann G., Hütter R. Isolation and characterization of an extrachromosomal element from Nocardia mediterranei. Plasmid. 1985 Sep;14(2):126–133. doi: 10.1016/0147-619x(85)90072-1. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Cohen S. N. Plasmid formation in Streptomyces: excision and integration of the SLP1 replicon at a specific chromosomal site. Mol Gen Genet. 1984;196(3):429–438. doi: 10.1007/BF00436190. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Cohen S. N. Structural analysis of plasmid and chromosomal loci involved in site-specific excision and integration of the SLP1 element of Streptomyces coelicolor. J Bacteriol. 1986 Jun;166(3):999–1006. doi: 10.1128/jb.166.3.999-1006.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernodet J. L., Simonet J. M., Guérineau M. Plasmids in different strains of Streptomyces ambofaciens: free and integrated form of plasmid pSAM2. Mol Gen Genet. 1984;198(2):35–41. doi: 10.1007/BF00328697. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Simonet J. M., Boccard F., Pernodet J. L., Gagnat J., Guérineau M. Excision and integration of a self-transmissible replicon of Streptomyces ambofaciens. Gene. 1987;59(1):137–144. doi: 10.1016/0378-1119(87)90274-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yi-guang W., Davies J. E., Hutchinson C. R. Plasmid DNA in the erythromycin producing microorganism, Streptomyces erythreus NRRL 2338. J Antibiot (Tokyo) 1982 Mar;35(3):335–342. doi: 10.7164/antibiotics.35.335. [DOI] [PubMed] [Google Scholar]








