Abstract
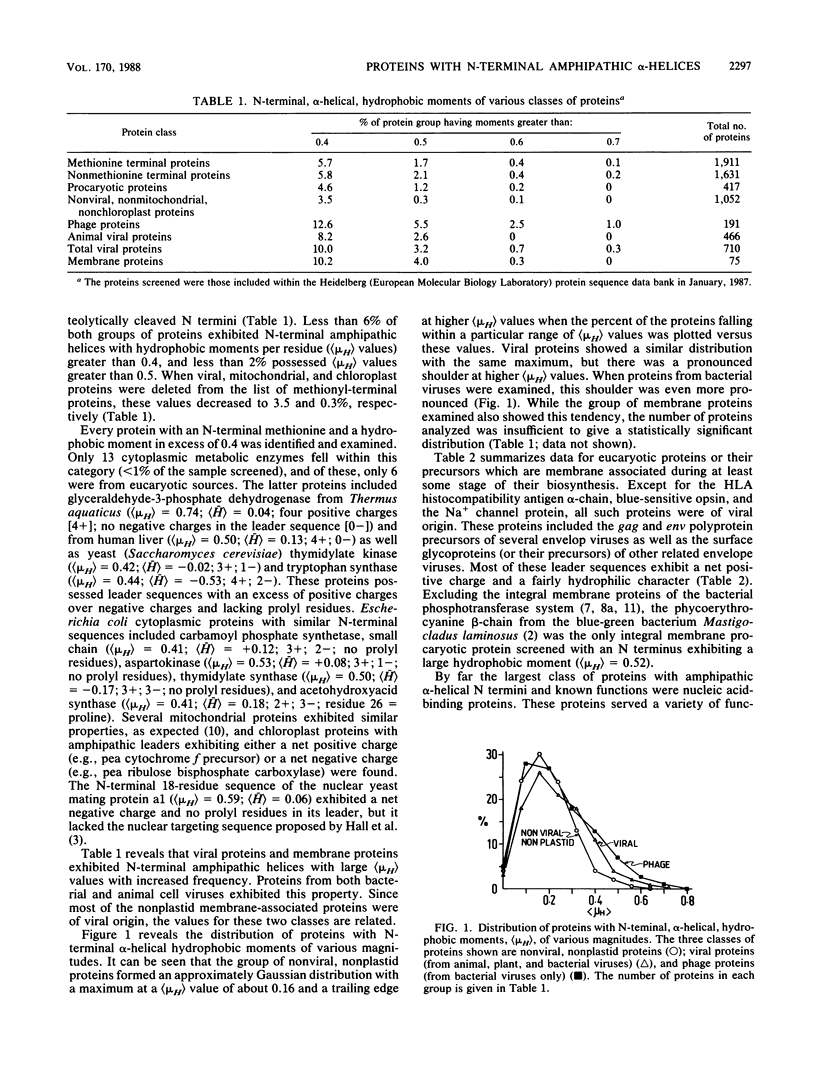
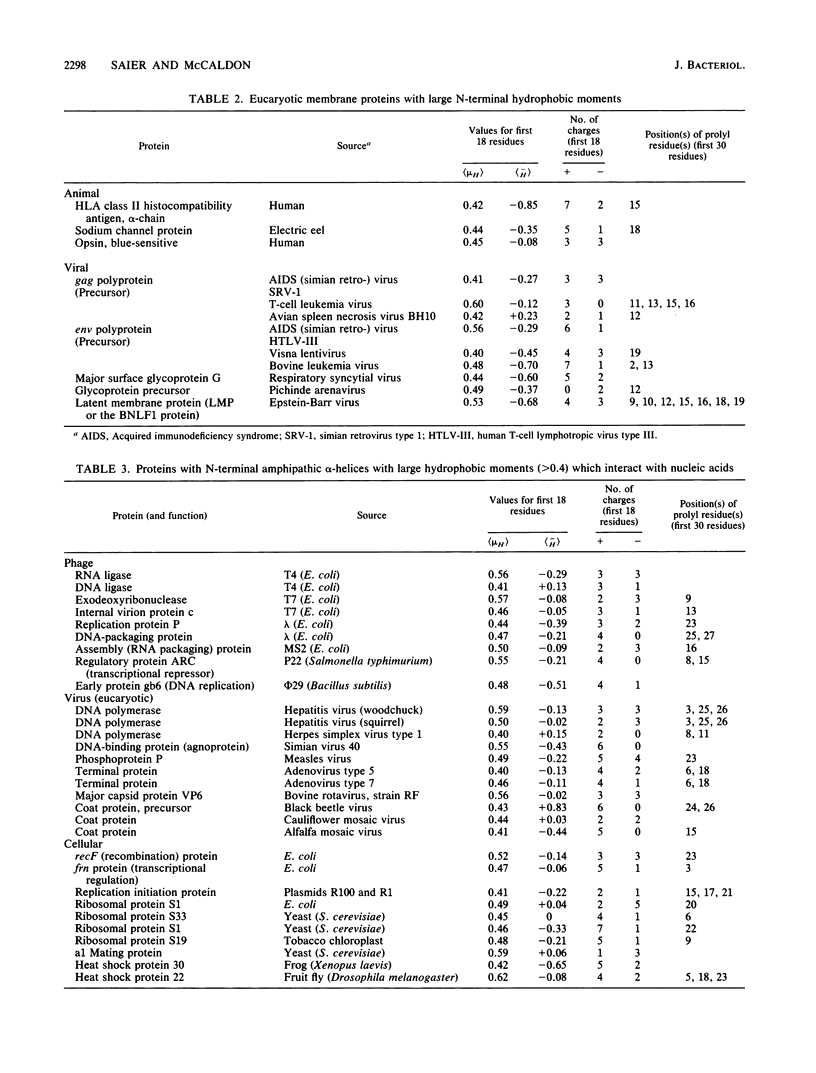
A total of 1,911 proteins with N-terminal methionyl residues were computer screened for potential N-terminal alpha-helices with strong amphipathic character. By the criteria of D. Eisenberg (Annu. Rev. Biochem. 53:595-623, 1984), only 3.5% of nonplastid, nonviral proteins exhibited potential N-terminal alpha-helices, 18 residues in length, with hydrophobic moment values per amino acyl residue ([muH]) in excess of 0.4. By contrast, 10% of viral proteins exhibited corresponding [muH] values in excess of 0.4. Of these viral proteins with known functions, 55% were found to interact functionally with nucleic acids, 30% were membrane-interacting proteins or their precursors, and 15% were structural proteins, primarily concerned with host cell interactions. These observations suggest that N-terminal amphipathic alpha-helices of viral proteins may (i) function in nucleic acid binding, (ii) facilitate membrane insertion, and (iii) promote host cell interactions. Analyses of potential amphipathic N-terminal alpha-helices of cellular proteins are also reported, and their significance to organellar or envelope targeting is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Füglistaller P., Suter F., Zuber H. The complete amino-acid sequence of both subunits of phycoerythrocyanin from the thermophilic cyanobacterium Mastigocladus laminosus. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):691–712. doi: 10.1515/bchm2.1983.364.1.691. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Hereford L., Herskowitz I. Targeting of E. coli beta-galactosidase to the nucleus in yeast. Cell. 1984 Apr;36(4):1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Suda K., Oppliger W., Schatz G. The first twelve amino acids (less than half of the pre-sequence) of an imported mitochondrial protein can direct mouse cytosolic dihydrofolate reductase into the yeast mitochondrial matrix. EMBO J. 1985 Aug;4(8):2061–2068. doi: 10.1002/j.1460-2075.1985.tb03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Soltanifar N., Goldschmidt-Clermont M., Rochaix J. D., Schatz G. The cleavable pre-sequence of an imported chloroplast protein directs attached polypeptides into yeast mitochondria. EMBO J. 1986 Jun;5(6):1343–1350. doi: 10.1002/j.1460-2075.1986.tb04365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Yamada M., Erni B., Suda K., Lengeler J., Ebner R., Argos P., Rak B., Schnetz K., Lee C. A. Sugar permeases of the bacterial phosphoenolpyruvate-dependent phosphotransferase system: sequence comparisons. FASEB J. 1988 Mar 1;2(3):199–208. doi: 10.1096/fasebj.2.3.2832233. [DOI] [PubMed] [Google Scholar]
- Schekman R. Protein localization and membrane traffic in yeast. Annu Rev Cell Biol. 1985;1:115–143. doi: 10.1146/annurev.cb.01.110185.000555. [DOI] [PubMed] [Google Scholar]
- Yamada M., Saier M. H., Jr Glucitol-specific enzymes of the phosphotransferase system in Escherichia coli. Nucleotide sequence of the gut operon. J Biol Chem. 1987 Apr 25;262(12):5455–5463. [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Segrest J. P. The leader peptides from bacteriorhodopsin and halorhodopsin are potential membrane-spanning amphipathic helices. FEBS Lett. 1987 Mar 23;213(2):238–240. doi: 10.1016/0014-5793(87)81497-7. [DOI] [PubMed] [Google Scholar]



