Abstract
Background
Obstructive sleep apnoea (OSA) is a common and potentially reversible cause of systemic hypertension. The mechanisms whereby OSA leads to hypertension and the effects of treatment on arterial function, however, are not well established. Microvascular arterial endothelial and smooth muscle function was assessed in subjects with OSA before and after treatment with continuous positive airways pressure (CPAP).
Methods
Ten subjects of mean (SE) age 49 (8) years with at least moderately severe OSA had detailed forearm vascular reactivity studies before and after 3 months of CPAP treatment. The systemic circulation was assessed by measuring brachial artery pressure, flow and resistance responses to intra‐arterial infusions of acetylcholine (ACh; an endothelium dependent vasodilator), sodium nitroprusside (SNP; an endothelium independent vasodilator), l‐NMMA (a nitric oxide (NO) antagonist), and l‐arginine (the substrate for NO).
Results
Before CPAP, ACh and SNP infusions increased forearm blood flow in a dose dependent manner (p<0.01). After CPAP, endothelium dependent dilation to ACh was significantly increased (434 (23)% of baseline after CPAP v 278 (20)% before CPAP, p<0.001), whereas SNP induced dilation was unchanged. Resting NO production was higher after CPAP, evidenced by a significantly greater reduction in basal flow by l‐NMMA (p = 0.05). l‐Arginine reversed the effect of l‐NMMA in all cases.
Conclusion
In patients with OSA, treatment with CPAP improves baseline endothelial NO release and stimulates endothelium dependent vasorelaxation in the systemic circulation. This is a potential mechanism for improving systemic and vascular function in patients with OSA treated with CPAP.
Keywords: hypertension, atherosclerosis, nitric oxide, obstructive sleep apnoea, continuous positive airway pressure
Obstructive sleep apnoea (OSA) is a common disease associated with a significantly increased risk of systemic hypertension.1,2 Moderate to severe OSA, with an apnoea hypopnoea index (AHI) of >15/hour, affects 4% of middle aged women and 9% of middle aged men.3 About 50% of individuals with OSA have systemic hypertension.4 OSA is characterised by repetitive airway occlusion resulting in cyclical surges of hypoxia which may occur hundreds of times a night. In addition, OSA is associated with heart failure,5 stroke,6,7 and coronary artery disease.8,9 The mechanisms that explain the relationship between OSA and daytime systemic hypertension and the ways to reduce OSA related vascular risk are unknown.10
Endothelial dysfunction, a key early event in hypertension and atherosclerosis, has been implicated as a possible mechanism linking the acute cyclical vascular stresses during sleep in OSA and the increased prevalence of chronic vascular diseases. We therefore hypothesised that nocturnal continuous positive airway pressure (CPAP), which alleviates airway obstruction, might improve systemic endothelial function.
Methods
Subjects
Consecutive consenting patients with newly diagnosed untreated moderate to severe OSA (AHI >15/hour) were enrolled from the Royal Prince Alfred Sleep Disorders Clinic, Sydney, Australia. Exclusion criteria included cigarette smoking, systemic hypertension, bleeding disorders, needle phobias, any regular vasoactive or antioxidant medications, and previous failed attempts at CPAP therapy.
Twelve subjects met these inclusion criteria and underwent baseline (pre‐CPAP) forearm vascular reactivity studies. Of these, two were unable to tolerate CPAP therapy and therefore 10 were invited to complete the second study. In all cases the study procedures were uncomplicated.
Institutional ethics committee approval was obtained for this study and written informed consent obtained from all subjects.
CPAP treatment
The subjects were autotitrated and treated with an automated CPAP device (Autoset T, ResMed, Sydney, Australia). CPAP compliance was monitored nightly throughout the study, with adequate compliance prospectively defined as being a mean of 5 hours usage per night with a reduction of obstructive episodes to AHI <5 for 3 months.
Measurements
During the vascular studies, ECG, heart rate and arterial blood pressure were measured continuously (Hewlett Packard, USA). Forearm blood flow was measured by strain gauge plethysmography (EC, Hokanson), in which stretch of a mercury in Silastic strain gauge correlates to increase in the volume of the forearm which is proportional to forearm blood flow.11 Cholesterol was measured by an automated enzymatic analyser (Hitachi 917, Japan; Roche Diagnostics, USA).
Study protocol
All subjects fasted from midnight before the experimental procedure and were studied in the morning in the supine position. The room was kept quiet and at a constant temperature (22–24°C). A 20 G arterial line (Arrow RA‐04120, Germany) was inserted by the Seldinger technique into the brachial artery of the non‐dominant arm of each subject under sterile conditions with local anaesthetic. Blood was drawn for analysis and the cannula was connected to heparinised saline 1000 units in 500 ml (Baxter) at an infusion rate of 60 ml/hour. The subjects were then rested for 30 minutes before initial baseline measurements.
The vasoactive drugs infused were acetylcholine (ACh; a vasodilator that stimulates the release of nitric oxide (NO) and other dilator substances from the endothelium), sodium nitroprusside (SNP; an endothelium independent vasodilator), l‐monomethylarginine (l‐NMMA; a competitive antagonist of NO synthesis), and l‐arginine (the physiological substrate for NO synthesis). Subjects were randomly assigned to either ACh or SNP as the first drug infusion.
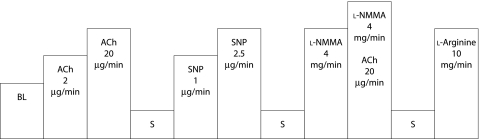
Serial infusions were made into the brachial circulation via the arterial line in the following sequence using a syringe pump (Terumo STC‐521, Tokyo, Japan): (1) a 4 minute control infusion (0.9% saline); (2) two consecutive 4 minutes of either 2 and 20 μg/min ACh or SNP; 1 and 2.5 μg/min; (3) a repeat control infusion; (4) two 4 minute infusions of the drug not infused in step 2, either ACh or SNP; (5) a repeat control infusion; (6) a 4 minute infusion of l‐NMMA (4 mg/min); (7) l‐NMMA 4 mg/min with ACh 20 μg/min; and (8) a 4 minute infusion of l‐arginine (10 mg/min) (fig 1). All measurements were made with the subject awake. Forearm blood flow is expressed as a percentage of the control forearm blood flow during the baseline infusion of saline. Forearm blood flow in the contralateral arm was also measured throughout to exclude effects from external stimuli.
Figure 1 Infusion protocol for all studies. The order of ACh and SNP infusions were randomised. Infusion rates for all substances were 1 ml/min. BL, baseline blood flow measurements with infusion of 1 ml/min 0.9% saline; S, 0.9% saline infusion; ACh, acetylcholine; SNP, sodium mitroprusside; l‐NMMA, NG‐monomethyl‐l‐arginine.
Analysis of data
All analyses were conducted by observers blinded to the subject identity and whether the study was conducted before or after CPAP treatment. Forearm blood flow was expressed as ml/min per 100 ml forearm volume. Changes in blood flow were reported as percentage change from baseline.
The results are expressed as mean (SE). Differences in resistance vessel dilation to drug infusions were determined by two way ANOVA with repeated measures (SPSS version 9). Differences between subject characteristics before and after CPAP were determined by paired Student's t tests. A two sided p value of ⩽0.05 was considered statistically significant.
Results
All 10 subjects were healthy non‐smokers of mean (SE) age 49 (8) years. Nine of the subjects were men. The female subject was post‐menopausal. All subjects had at least moderate OSA with an AHI of 39/hour (range 15–104). The subjects were not on any regular medications and did not start any medications during the study.
Baseline study (pre‐CPAP)
Before CPAP, systemic blood pressure was 122/80 mm Hg (SE 11/6). The body mass index (BMI) was in the obese range: 31 kg/m2 (range 24–41), as is frequently seen in the OSA population. Total cholesterol, LDL, and HDL were all within normal limits. Between study visits there were no significant changes in resting blood pressure or heart rate, BMI, or the lipid profile of the subjects (table 1).
Table 1 Patient characteristics.
| Characteristic | Pre‐CPAP | Post‐CPAP |
|---|---|---|
| Age (years) | 49 (8) | 49 (8) |
| AHI (/hour) | 39 (15–104) | 4.4 (2.1–6.1)* |
| BMI (kg/m2) | 31 (24–41) | 31 (25–41) |
| Systolic BP (mm Hg) | 122 (11) | 125 (13) |
| Diastolic BP (mm Hg) | 80 (6) | 76 (12) |
| Total cholesterol (mmol/l) | 4.9 (0.9) | 4.6 (0.9) |
Values expressed as mean (SE) or median (range).
AHI, apnoea hypopnea index; BMI, body mass index; BP, blood pressure.
*p<0.01.
In response to ACh there was a dose dependent increase in brachial artery flows compared with the baseline value (p<0.01). There was also a dose dependent increase in flow to SNP (p<0.01). By contrast, there was no significant change in flow to l‐NMMA. Co‐infusion of l‐NMMA partially inhibited the increase in forearm blood flow with ACh (p<0.01, fig 1).
Post‐CPAP study
AHI was reduced to 4.4/hour (range 2.1–6.1) on successful CPAP (p<0.01). Baseline forearm blood flow was not significantly changed, nor was the arterial blood pressure (125/76 mm Hg (SE 13/12).
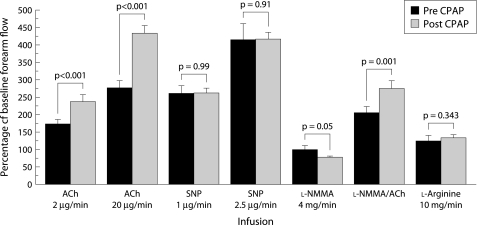
In response to ACh there was a significantly greater increase in flow compared with pre‐CPAP (434 (23)% v 278 (20)%, p<0.001; table 2). This CPAP related increase was observed with both low and high dose ACh. By contrast, SNP responses were similar to pre‐CPAP values (fig 1). After CPAP, l‐NMMA induced vasoconstriction and a greater reduction in the brachial artery flow compared with pre‐CPAP values (p = 0.05; fig 2). Co‐infusion of l‐NMMA reduced the high dose ACh related increase in forearm blood flow, although the flow remained greater than the pre‐CPAP co‐infusion flow values (p<0.001). l‐Arginine reversed the l‐NMMA related constriction, consistent with an inhibitory effect of l‐NMMA on the NO pathway (fig 2).
Table 2 Forearm vascular reactivity before and after CPAP (expressed as a % baseline flow).
| ACh low | ACh high | SNP low | SNP high | l‐NMMA high | ACh/l‐NMMA | l‐Arg | |
|---|---|---|---|---|---|---|---|
| Before CPAP | |||||||
| Subject 1 | 167 | 209 | 624 | 1250 | 164 | 149 | 80 |
| Subject 2 | 132 | 296 | 281 | 357 | 67 | 60 | 57 |
| Subject 3 | 362 | 474 | 372 | 650 | 95 | 431 | 159 |
| Subject 4 | 141 | 156 | 149 | 222 | 113 | 124 | 138 |
| Subject 5 | 167 | 572 | 376 | 476 | 94 | 420 | 128 |
| Subject 6 | 135 | 168 | 151 | 214 | 114 | 137 | 135 |
| Subject 7 | 122 | 183 | 224 | 285 | 63 | 174 | 135 |
| Subject 8 | 300 | 442 | 264 | 354 | 62 | 372 | 161 |
| Subject 9 | 87 | 163 | 134 | 194 | 67 | 106 | 125 |
| Subject 10 | 106 | 163 | 88 | 101 | 78 | 104 | 111 |
| After CPAP | |||||||
| Subject 1 | 122 | 289 | 188 | 368 | 62 | 159 | 176 |
| Subject 2 | 228 | 369 | 249 | 340 | 94 | 227 | 146 |
| Subject 3 | 508 | 764 | 403 | 538 | 82 | 470 | 167 |
| Subject 4 | 495 | 504 | 336 | 561 | 103 | 138 | 130 |
| Subject 5 | 190 | 661 | 472 | 571 | 103 | 520 | 147 |
| Subject 6 | 130 | 267 | 291 | 393 | 66 | 59 | 61 |
| Subject 7 | 131 | 253 | 382 | 523 | 80 | 183 | 122 |
| Subject 8 | 248 | 411 | 153 | 337 | 70 | 278 | 136 |
| Subject 9 | 206 | 412 | 153 | 293 | 70 | 273 | 137 |
| Subject 10 | 105 | 410 | 207 | 260 | 67 | 459 | 131 |
ACh, acetylcholine; SNP, sodium nitroprusside; l‐NMMA, NG‐monomethyl‐l‐arginine; l‐Arg, l‐arginine.
Figure 2 Forearm blood flow responses before and after CPAP showing significantly enhanced responses to ACh alone or ACh with l‐NMMA after CPAP than before (p<0.001). SNP responses were unchanged with CPAP treatment. The vasoconstrictor response to l‐NMMA was significantly greater after CPAP than before (p = 0.05), consistent with an enhanced release of nitric oxide from the vascular endothelium.
There was no correlation between severity of noctural hypoxaemia (r = 0.18, p = 0.62), AHI (r = 0.61, p = 0.062), or CPAP compliance (r = 0.71, p = 0.85) and the magnitude of the change in vascular function with treatment. This finding is, however, consistent with the small size of the study.
Discussion
OSA is a major and underdiagnosed risk factor for hypertension and its complications.1,2 In this study we found that CPAP, an effective treatment for OSA, significantly improves microvascular function in the systemic circulation of OSA subjects. Specifically, CPAP treatment of OSA enhanced endothelial NO release in the forearm microcirculation (as evidenced by enhanced l‐NMMA related vasoconstriction) and improved stimulated endothelium dependent vasodilatation (as shown by greater ACh vasodilator responses). This improvement in vasodilatation after ACh was only partially inhibited by co‐infusion of l‐NMMA, indicating that not only NO endothelium dependent pathways but also non‐NO pathways were enhanced. These significant changes in vascular reactivity were observed after only 3 months of effective CPAP treatment. By contrast, the responses to the endothelium independent dilator SNP did not differ before and after CPAP treatment, consistent with improved endothelial but not smooth muscle vasodilator function.
It has previously been shown that subjects with OSA have impaired systemic endothelial function compared with control subjects. Carlson et al12 found impairment of the ACh response in normotensive and hypertensive OSA subjects compared with those without OSA loosely matched for age, sex and weight. In this study impairment in the SNP response was also reported in the hypertensive OSA subjects. However, Kato et al13 recently compared the vascular responses in eight subjects with OSA with a tightly matched control group on no medications and found that ACh responses were impaired but there was no difference in the SNP response.
Several mechanisms have been proposed to explain the reduction in endothelial responsiveness in patients with OSA. Animal studies have shown that intermittent hypoxia plays a key role in the development of persistent daytime endothelial dysfunction. Sprague‐Dawley rats exposed to intermittent hypoxia have less in vivo responsiveness to increasing doses of ACh than control rats. There is also significantly greater vasoconstriction to l‐NAME (an NO antagonist) in intermittently hypoxic rats, suggesting impairment of basal NO production.14 Human studies have also pointed to hypoxia as a possible cause of endothelial impairment; abnormal vascular responses have been observed in OSA patients exposed to acute isocapnic hypoxia.15
Possible mechanisms of hypoxia induced impairment of endothelial function might include direct damage to the endothelium or altered biosynthesis of NO from l‐arginine, which is an oxygen dependent process.16 Such hypoxia induced endothelial impairment may occur in isolation or it may be additive to other acute effects of apnoea such as cyclical surges of blood pressure.
Our data have shown (for the first time to our knowledge) that microvascular endothelial vasodilator dysfunction in subjects with OSA is reversible with appropriate long term treatment; indeed, basal and stimulated endothelial vasodilator release are significantly enhanced. Previous studies have shown that CPAP might reduce the acute physiological effects of apnoeas in OSA, including cyclical hypoxia, surges in blood pressure and sympathetic nerve activity,17,18,19 but have not addressed longer term effects on vascular physiology. Imadojemu et al20 found an improvement in forearm vasoreactivity in response to a limited non‐invasive reactivity study after 2 weeks of CPAP therapy.
Consistent with our findings are the results from two studies on the effect of CPAP on plasma levels of NO derivatives in subjects with OSA. Ip et al21 reported reduced serum nitrite and nitrate levels in subjects with moderate to severe OSA compared with matched controls, which returned to normal levels after one night of CPAP treatment. Schulz et al22 also found increased serum NO derivatives in subjects with OSA after short term CPAP treatment, and this increase in NO derivatives was maintained at long term follow up. CPAP treatment also improves hypertension control, both at night and during the day,23 by an average of 10 mm Hg (systolic, mean and diastolic), which may in part be explained by the improvement in microvascular endothelial vascular function.24
Although most studies of endothelial function in OSA have concentrated on the microvasculature—the level of the systemic circulation considered most relevant in the pathogenesis of hypertension25—other groups have recently reported studies of endothelial function in peripheral conduit vessels such as the brachial artery. Both Kraiczi et al26 and Ip et al27 have found impaired flow mediated dilation (FMD) in OSA subjects consistent with a predisposition to atherosclerosis; Ip et al also reported a beneficial effect of CPAP on FMD (although no mechanistic data regarding the role of NO were available).
One of the limitations of our study is its relatively small size due to the invasive nature of the protocol and the restrictions of our enrolment criteria. The study was not therefore able to find a correlation with the severity of OSA and the degree of endothelial impairment. Furthermore, we elected not to perform these invasive studies on a control group of normal subjects without OSA in whom forearm vascular responses to ACh, SNP, and l‐NMMA have been extensively published previously with consistent results.13,25,28
Kato et al found a similar dose dependent increase in forearm blood flow in untreated OSA patients in response to brachial artery infusions of ACh and SNP, with the increase in endothelium dependent vasodilatation to ACh blunted compared with normal controls, indicating impaired microvascular endothelial function.13 For our study, each patient was their own control for the vascular responses after CPAP and no difference was found in the characteristics of the subjects before and after CPAP.
Finally, we elected to study normotensive rather than hypertensive subjects with OSA. This was to allow study of the underlying vascular mechanisms in the absence of any confounding effects of antihypertensive medications, many of which are known to influence endothelial function per se29 even several half‐lives after discontinuation.30 Furthermore, as CPAP is known to reduce blood pressure in hypertensive OSA subjects,24 we also wished to avoid the possible confounding effects of blood pressure lowering itself on systemic endothelial and smooth muscle dependent vascular reactivity.
In conclusion, treatment of OSA with CPAP improves systemic vascular endothelial function. OSA has been implicated in the pathogenesis of hypertension, cardiovascular disease, heart failure, and stroke, all of which are associated with impaired endothelial responses. CPAP treatment may therefore provide an opportunity to reduce the vascular risk attributable to OSA; long term end point studies will be needed to address this important possibility.
Abbreviations
ACh - acetylcholine
AHI - apnoea hypopnoea index
BMI - body mass index
CPAP - continuous positive airway pressure
l‐NMMA - NG‐monomethyl‐l‐arginine
NO - nitric oxide
OSA - obstructive sleep apnoea
SNP - sodium nitroprusside
Footnotes
Dr Lattimore is supported by a Cardiac Society of Australia and New Zealand Research Scholarship. This work was supported in part by a grant‐in‐aid from the National Heart Foundation of Australia.
A/Prof Ian Wilcox is a minor shareholder in ResMed, Australia.
References
- 1.Peppard P E, Young T, Palta M.et al Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med 20003421378–1384. [DOI] [PubMed] [Google Scholar]
- 2.Nieto F J, Young T B, Lind B K.et al Association of sleep‐disordered breathing, sleep apnea, and hypertension in a large community‐based study. Sleep Heart Health Study. JAMA 20002831829–1836. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J.et al The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med 19933281230–1235. [DOI] [PubMed] [Google Scholar]
- 4.Parati G, Ongaro G, Bonsignore M R.et al Sleep apnoea and hypertension. Curr Opin Nephrol Hypertens 200211201–214. [DOI] [PubMed] [Google Scholar]
- 5.Javaheri S, Parker T J, Liming J D.et al Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation 1998972154–2159. [DOI] [PubMed] [Google Scholar]
- 6.Dyken M E, Somers V K, Yamada T, e al Investigating the relationship between stroke and obstructive sleep apnea. Stroke 199627401–407. [DOI] [PubMed] [Google Scholar]
- 7.Wessendorf T E, Teschler H, Wang Y M.et al Sleep‐disordered breathing among patients with first‐ever stroke. J Neurol 200024741–47. [DOI] [PubMed] [Google Scholar]
- 8.Hung J, Whitford E G, Parsons R W.et al Association of sleep apnoea with myocardial infarction in men. Lancet 1990336261–264. [DOI] [PubMed] [Google Scholar]
- 9.Peker Y, Kraiczi H, Hedner J.et al An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J 199914179–184. [DOI] [PubMed] [Google Scholar]
- 10.Lattimore J D, Celermajer D S, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol 2003411429–1437. [DOI] [PubMed] [Google Scholar]
- 11.Hokanson D E, Sumner D S, Strandness D E., Jr An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans Biomed Eng 19752225–29. [DOI] [PubMed] [Google Scholar]
- 12.Carlson J T, Rangemark C, Hedner J A. Attenuated endothelium‐dependent vascular relaxation in patients with sleep apnoea. J Hypertens 199614577–584. [DOI] [PubMed] [Google Scholar]
- 13.Kato M, Roberts‐Thomson P, Phillips B G.et al Impairment of endothelium‐dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 20001022607–2610. [DOI] [PubMed] [Google Scholar]
- 14.Tahawi Z, Orolinova N, Joshua I G.et al Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J Appl Physiol 2001902007–2013. [DOI] [PubMed] [Google Scholar]
- 15.Remsburg S, Launois S H, Weiss J W. Patients with obstructive sleep apnea have an abnormal peripheral vascular response to hypoxia. J Appl Physiol 1999871148–1153. [DOI] [PubMed] [Google Scholar]
- 16.Dean R T, Wilcox I. Possible atherogenic effects of hypoxia during obstructive sleep apnea. Sleep 199316(8 Suppl)S15–S22. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan C E, Issa F G, Berthon‐Jones M.et al Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 19811862–865. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox I, Grunstein R R, Hedner J A.et al Effect of nasal continuous positive airway pressure during sleep on 24‐hour blood pressure in obstructive sleep apnea. Sleep 199316539–544. [DOI] [PubMed] [Google Scholar]
- 19.Narkiewicz K, Kato M, Phillips B G.et al Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 19991002332–2335. [DOI] [PubMed] [Google Scholar]
- 20.Imadojemu V A, Gleeson K, Quraishi S A.et al Impaired vasodilator responses in obstructive sleep apnea are improved with continuous positive airway pressure therapy. Am J Respir Crit Care Med 2002165950–953. [DOI] [PubMed] [Google Scholar]
- 21.Ip M S, Lam B, Chan L Y.et al Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med 20001622166–2171. [DOI] [PubMed] [Google Scholar]
- 22.Schulz R, Schmidt D, Blum A.et al Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax 2000551046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepperell J C T, Ramdassingh‐Dow S, Crosthawaite N.et al Ambulatory blood pressure after theraputic and subtherapeutic nasal positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002359204–210. [DOI] [PubMed] [Google Scholar]
- 24.Becker H F, Jerrentrup A, Ploch T.et al Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 200310768–73. [DOI] [PubMed] [Google Scholar]
- 25.Taddei S, Virdis A, Mattei P.et al Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 1995911981–1987. [DOI] [PubMed] [Google Scholar]
- 26.Kraiczi H, Caidahl K, Samuelsson A.et al Impairment of vascular endothelial function and left ventricular filling: association with the severity of apnea‐induced hypoxemia during sleep. Chest 20011191085–1091. [DOI] [PubMed] [Google Scholar]
- 27.Ip M S, Tse H F, Lam B.et al Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 2004169348–353. [DOI] [PubMed] [Google Scholar]
- 28.Panza J A, Quyyumi A A, Brush J E., Jret al Abnormal endothelium‐dependent vascular relaxation in patients with essential hypertension. N Engl J Med 199032322–27. [DOI] [PubMed] [Google Scholar]
- 29.Kalinowski L, Dobrucki L W, Szczepanska‐Konkel M.et al Third‐generation beta‐blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation 20031072747–2752. [DOI] [PubMed] [Google Scholar]
- 30.Mancini G B, Henry G C, Macaya C.et al Angiotensin‐converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation 199694258–265. [DOI] [PubMed] [Google Scholar]