Abstract
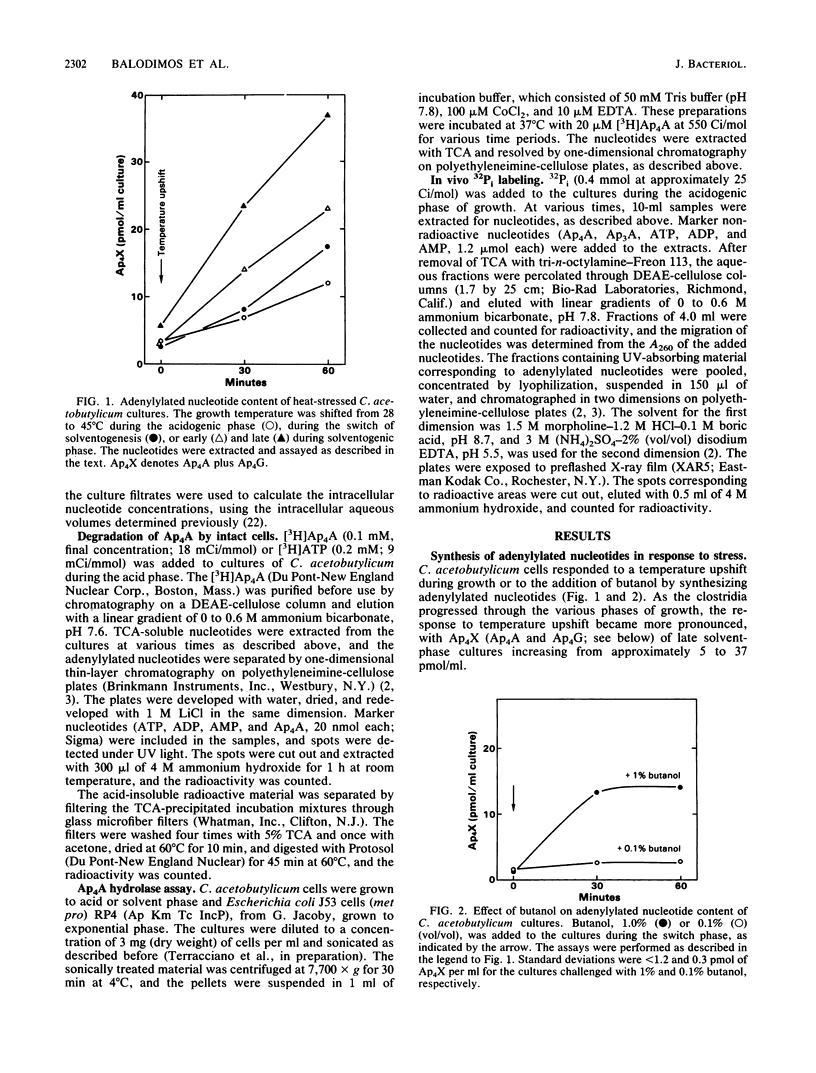
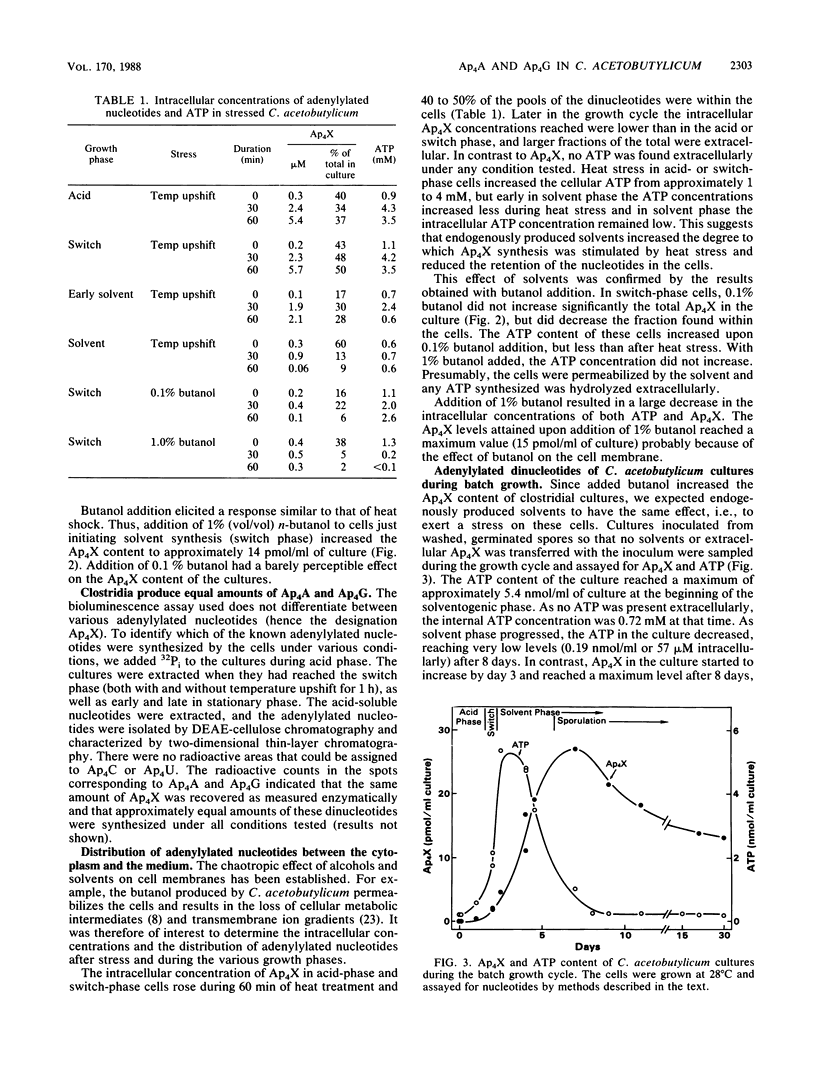
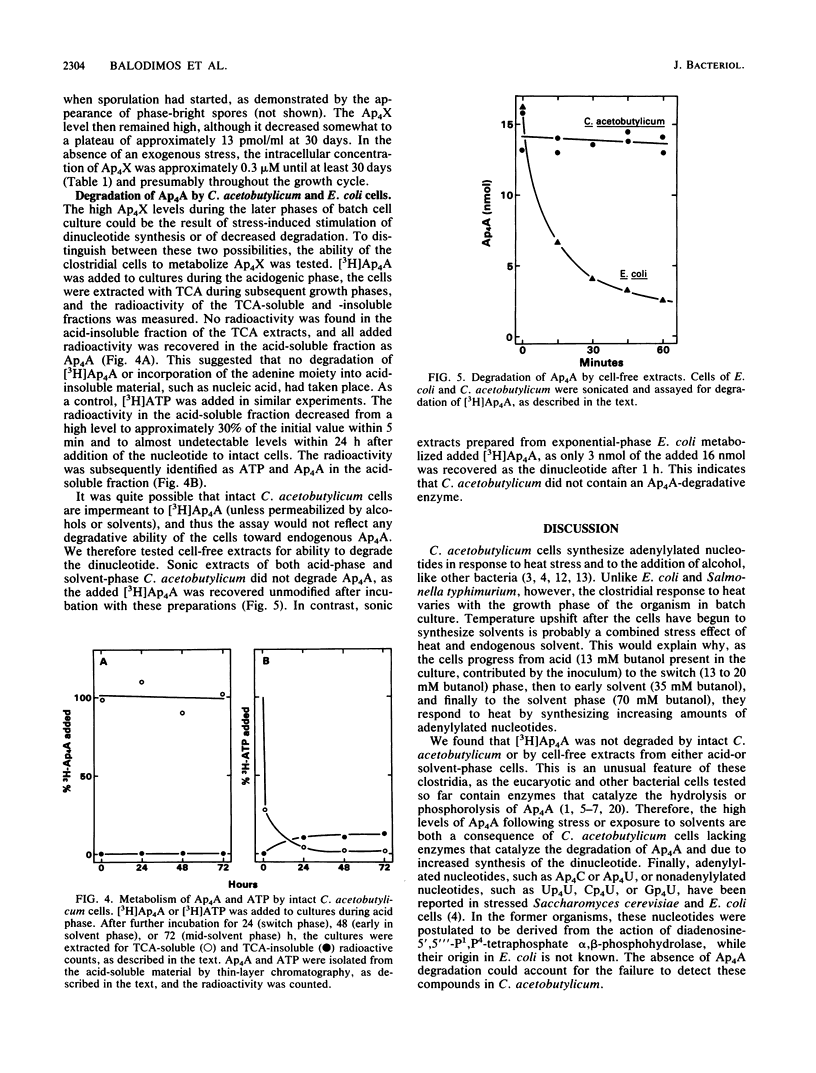
In response to the stresses imposed by temperature upshift or addition of butanol, Clostridium acetobutylicum cultures accumulated diadenosine-5',5'''-P1,P4-tetraphosphate (Ap4A) and adenosine 5'-P1,P4-tetraphospho-5'-guanosine (Ap4G) to high levels. The two adenylylated nucleotides were also accumulated in batch culture in the absence of imposed stresses when the clostridia switched from the acidogenic phase of growth to the solventogenic phase. Most of the adenylylated nucleotides were extracellular. The intracellular concentrations of these compounds were low throughout batch growth and in cells stressed by added butanol. In contrast to other procaryotes, these clostridia did not possess enzymes to degrade the dinucleotides, as shown with both intact cells and cell-free preparations. Our findings are consistent with the hypothesis that endogenously produced solvents are stressful to the cells, stimulating the synthesis of adenylylated nucleotides. The nucleotides accumulate extracellularly because they cannot be degraded and because the cell membranes are permeabilized by the solvents produced.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes L. D., Culver C. A. Isolation and characterization of diadenosine 5',5"'-P1,P4-tetraphosphate pyrophosphohydrolase from Physarum polycephalum. Biochemistry. 1982 Nov 23;21(24):6123–6128. doi: 10.1021/bi00267a015. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Coste H., Brevet A., Plateau P., Blanquet S. Non-adenylylated bis(5'-nucleosidyl) tetraphosphates occur in Saccharomyces cerevisiae and in Escherichia coli and accumulate upon temperature shift or exposure to cadmium. J Biol Chem. 1987 Sep 5;262(25):12096–12103. [PubMed] [Google Scholar]
- Guranowski A., Blanquet S. Phosphorolytic cleavage of diadenosine 5',5'''-P1,P4-tetraphosphate. Properties of homogeneous diadenosine 5',5'''-P1,P4-tetraphosphate alpha, beta-phosphorylase from Saccharomyces cerevisiae. J Biol Chem. 1985 Mar 25;260(6):3542–3547. [PubMed] [Google Scholar]
- Guranowski A., Jakubowski H., Holler E. Catabolism of diadenosine 5',5"'-P1,P4-tetraphosphate in procaryotes. Purification and properties of diadenosine 5',5"'-P1,P4-tetraphosphate (symmetrical) pyrophosphohydrolase from Escherichia coli K12. J Biol Chem. 1983 Dec 25;258(24):14784–14789. [PubMed] [Google Scholar]
- Hutkins R. W., Kashket E. R. Phosphotransferase Activity in Clostridium acetobutylicum from Acidogenic and Solventogenic Phases of Growth. Appl Environ Microbiol. 1986 May;51(5):1121–1123. doi: 10.1128/aem.51.5.1121-1123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhn M., Albert W., Grummt F. Diadenosine tetraphosphate hydrolase from mouse liver. Purification to homogeneity and partial characterization. J Biol Chem. 1982 Mar 25;257(6):3003–3006. [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., van der Westhuizen A., Long S., Allcock E. R., Reid S. J., Woods D. R. Solvent Production and Morphological Changes in Clostridium acetobutylicum. Appl Environ Microbiol. 1982 Jun;43(6):1434–1439. doi: 10.1128/aem.43.6.1434-1439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Stoichiometry of the H+-ATPase of growing and resting, aerobic Escherichia coli. Biochemistry. 1982 Oct 26;21(22):5534–5538. doi: 10.1021/bi00265a024. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. Diadenosine 5',5"'-P1,P4-tetraphosphate and related adenylylated nucleotides in Salmonella typhimurium. J Biol Chem. 1983 Jun 10;258(11):6827–6834. [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- McLennan A. G., Prescott M. Diadenosine 5',5'"-P1,P4-tetraphosphate in developing embryos of Artemia. Nucleic Acids Res. 1984 Feb 10;12(3):1609–1619. doi: 10.1093/nar/12.3.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Ogilvie A. Determination of diadenosine tetraphosphate (Ap4A) levels in subpicomole quantities by a phosphodiesterase luciferin--luciferase coupled assay: application as a specific assay for diadenosine tetraphosphatase. Anal Biochem. 1981 Aug;115(2):302–307. doi: 10.1016/0003-2697(81)90009-9. [DOI] [PubMed] [Google Scholar]
- Plateau P., Fromant M., Blanquet S. Heat shock and hydrogen peroxide responses of Escherichia coli are not changed by dinucleoside tetraphosphate hydrolase overproduction. J Bacteriol. 1987 Aug;169(8):3817–3820. doi: 10.1128/jb.169.8.3817-3820.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateau P., Fromant M., Brevet A., Gesquière A., Blanquet S. Catabolism of bis(5'-nucleosidyl) oligophosphates in Escherichia coli: metal requirements and substrate specificity of homogeneous diadenosine-5',5'''-P1,P4-tetraphosphate pyrophosphohydrolase. Biochemistry. 1985 Feb 12;24(4):914–922. doi: 10.1021/bi00325a016. [DOI] [PubMed] [Google Scholar]
- Terracciano J. S., Kashket E. R. Intracellular Conditions Required for Initiation of Solvent Production by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):86–91. doi: 10.1128/aem.52.1.86-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano J. S., Schreurs W. J., Kashket E. R. Membrane H Conductance of Clostridium thermoaceticum and Clostridium acetobutylicum: Evidence for Electrogenic Na/H Antiport in Clostridium thermoaceticum. Appl Environ Microbiol. 1987 Apr;53(4):782–786. doi: 10.1128/aem.53.4.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. Diadenosine 5',5"'-P1,P4-tetraphosphate (Ap4A): its role in cellular metabolism. Anal Biochem. 1983 Oct 1;134(1):1–10. doi: 10.1016/0003-2697(83)90255-5. [DOI] [PubMed] [Google Scholar]



