Abstract
Background
Chronic obstructive pulmonary disease (COPD) is characterised by both airway inflammation and systemic changes. To elucidate the relationship between local and systemic inflammation, tumour necrosis factor α (TNFα) production by sputum cells and blood cells of patients with COPD and controls was compared and the effect of the extracellular matrix compound hyaluronan (HA) on TNFα release was studied.
Methods
Four study groups were included: 10 steroid free COPD patients, 8 steroid treated patients, 10 healthy smokers, and 11 healthy non‐smokers. Sputum cells and blood were incubated for 24 hours with or without lipopolysaccharide (LPS) in the absence or presence of HA (122 kDa or HMW fragment). TNFα was measured by ELISA.
Results
Sputum cells produced spontaneously high levels of TNFα but were unresponsive to LPS. Sputum cells from COPD patients (both steroid free and steroid treated) produced significantly less TNFα than cells from healthy non‐smoking subjects (p = 0.017 and p = 0.001, respectively). In contrast, blood cells produced TNFα only in response to LPS. No differences were observed in TNFα production by blood cells between the patient groups and the control groups. HA (both fragments) partially blocked LPS (1 ng/ml) induced TNFα release by blood cells from all study groups, whereas TNFα production by sputum cells was not influenced by HA.
Conclusion
These data indicate a difference between local and systemic TNFα production. Sputum cells of patients with COPD produced less TNFα than controls, which could contribute to impaired local defence. An inhibitory effect of HA on TNFα release in blood cells was observed which was similar in both patients and controls.
Keywords: chronic obstructive pulmonary disease, hyaluronan, inflammation, sputum cells, tumour necrosis factor α (TNFα)
Chronic obstructive pulmonary disease (COPD) is a complex heterogeneous respiratory disease characterised by the progressive development of airflow limitation that is largely irreversible. Airway inflammation is a key feature of COPD and is reasoned to play a pathogenic role.1 Influx of neutrophils and macrophages in the airway wall is related to airway obstruction in patients with severe COPD.2 Moreover, infiltration of CD8+ lymphocytes has been seen throughout the whole lung which is inversely related to forced expiratory volume in 1 second (FEV1).3,4 Progression of the disease is also associated with the presence of polymorphonuclear cells, macrophages, CD4 cells, and lymphocyte subtypes in the small airways.5 These inflammatory cells may be an important source of inflammatory mediators and proteases which can enhance inflammatory processes in COPD. In induced sputum from patients with COPD, increased levels of the chemokine interleukin (IL)‐8, the pro‐inflammatory cytokine tumour necrosis factor (TNF)α, and its soluble receptors (sTNF‐R) have been observed.6,7 Enhanced levels of inflammatory markers such as TNFα and the acute phase protein C‐reactive protein (CRP) have also been reported in the circulation,7,8 indicating a systemic inflammatory reaction. A clear association has been shown between circulating inflammatory mediators and other systemic effects of COPD such as tissue wasting and cardiovascular morbidity.9 Very little is known about the origin of the systemic inflammation in COPD. In a previous study we showed that levels of sputum and circulating inflammatory markers were not correlated, suggesting that the systemic inflammation in COPD is not due to an overflow of inflammatory mediators from the pulmonary compartment.7 In order to further elucidate the relationship between local and systemic inflammation, the production of TNFα by sputum cells and blood cells of COPD patients (either steroid free or steroid treated) versus smoking and non‐smoking control subjects was analysed.
Chronic pulmonary inflammation and lung injury are associated with damage, repair and remodelling of the extracellular matrix (ECM). Recently, we have demonstrated enhanced levels of the ECM compound hyaluronan (HA) in sputum supernatant of COPD patients.10 Moreover, in circulation of inflammatory diseases such as rheumatoid arthritis elevated HA levels were reported.11 HA is known to have pro‐inflammatory potential when present in small fragments, and to be anti‐inflammatory in high molecular size.12 Therefore, we also analysed the effect of HA on sputum and blood cell TNFα production and compared responsiveness of COPD versus control subjects to this ECM compound.
Methods
Study groups
COPD patients
Eighteen patients with moderate to severe COPD were recruited from the outpatient clinic and rehabilitation centre CHU‐Sart‐Tilman. A summary of their characteristics is shown in table 1. COPD was diagnosed according to the GOLD criteria—that is, post‐bronchodilator (400 µg inhaled sabutamol) FEV1/FVC ratio <70%. All the COPD subjects were life long heavy smokers (more than 15 pack years). They were all using long acting bronchodilators including tiotropium, formoterol, or salmeterol. Eight of the 18 patients were receiving inhaled corticosteroids (1000 µg fluticasone or 800 µg budesonide/day) and form the steroid treated group; the other patients were steroid free.
Table 1 Demographic and functional characteristics of study groups.
| Control subjects | COPD patients | |||
|---|---|---|---|---|
| Non‐smoking | Smoking | Steroid free | Steroid treated | |
| (n = 11) | (n = 10) | (n = 10) | (n = 8) | |
| Sex (M/F) | 6/5 | 7/3 | 8/2 | 6/2 |
| Age | 53 (42–69) | 51 (41–63) | 67 (38–74)*† | 58 (45–78) |
| Smoking behaviour | ||||
| Non/ex/current | 11/0/0 | 0/4/6* | 0/2/8* | 0/6/2* |
| Pack‐years | 0 | 23 (13–44)* | 45 (24–56)*† | 47 (15–60)*† |
| FEV1 (ml) | 3760 (2130–5670) | 3465 (2480–4840) | 1215 (890–3010)*† | 1155 (600–1410)*† |
| FEV1 (% predicted) | 109 (94–133) | 99 (87–121) | 41 (29–75)*† | 35 (23–62)*† |
| FVC (ml) | 4690 (2620–6550) | 4260 (2940–7030) | 2525 (1530–5350)*† | 1675 (1040–3090)*† |
| FVC (% predicted) | 120 (91–127) | 100 (89–143) | 59 (44–107)*† | 48 (29–72)*† |
| FEV1/FVC (%) | 80 (71–90) | 79 (71–90) | 53 (43–62)*† | 60 (42–68)*† |
| RV (% predicted) | ND | ND | 215 (141–242) | 227 (189–242) |
| TLC (% predicted) | ND | ND | 109 (104–146) | 120 (100–131) |
| BMI | 23.4 (21.2–31.6) | 24.3 (21.2–31.6) | 25.6 (21.0–29.4) | 25.9 (16.5–25.9) |
Values are expressed as median (range) or as absolute numbers.
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; RV, residual volume; TLC, total lung capacity; BMI, body mass index; ND, not determined.
Differences between groups were tested using the Kruskal‐Wallis H test and, if appropriate (p<0.05), subsequent analysis of subgroups was performed using Mann‐Whitney U test or the Fisher's exact test.
*p<0.05 v non‐smoking controls; †p<0.05 v smoking controls.
Control subjects
Twenty one control subjects were recruited through advertisement from the hospital staff of CHU Sart Tilman. Care was taken to recruit patients who matched the patients with COPD with respect to age and sex. Ten of the control subjects were current smokers with a mean of 23 pack years (table 1).
The protocol was approved by the local ethical committee and volunteers gave their signed informed consent.
Sputum induction and processing
After premedication with 400 µg inhaled salbutamol, sputum was induced by inhalation of a hypertonic saline solution (NaCl 4.5%) when FEV1 was >65% predicted and by isotonic saline (NaCl 0.9%) when FEV1 was <65% predicted. In order to improve bronchoprotection, additional amounts of salbutamol were delivered during the saline nebulisation itself as previously described.13 Saline aerosols were delivered by an ultrasonic nebuliser (Ultra‐Neb 2000, De Vilbiss, Somerset, USA) with an output of 1.5 ml/min. Each subject inhaled the aerosol for three consecutive periods of 5 minutes for a total time of 15 minutes. For safety reasons, the FEV1 was monitored every 5 minutes and the induction stopped when FEV1 fell by more than 20% from baseline.
Whole sputum was collected in a 50 ml polypropylene tube, weighed, and diluted 1:4 with phosphate buffered saline without Ca2+ and Mg2+ (DPBS), vortexed for 30 seconds and centrifuged at 800 g for 10 minutes at 4°C. The supernatant and cell pellet diluted in 20 ml of DPBS were filtered separately on double thickness gauze. The supernatant was stored at −80°C until analysed for TNFα and IL‐8. Cells were washed and suspended in 1 ml DPBS for total cell counts using a manual haemocytometer. The differential cell count was performed on cytospins stained with Diff‐Quick by counting 500 cells under a light microscope.
Sputum cell and whole blood cell stimulation
Sputum cells were obtained as reported above. In order to perform blood cell stimulation, heparinised whole blood was collected and diluted 1:20. Both sputum cells (4×105 non‐squamous cells/ml) and whole blood were dissolved in RPMI‐1640, supplemented with penicillin and streptomycin and 5% of heat inactivated fetal calf serum (FCS), and incubated for 24 hours at 37°C in plates coated with anti‐TNFα antibody as described below. Stimulation was performed in the absence or presence of lipopolysaccharide (LPS E coli, Sigma, St Louis, MO, USA) and in the absence or presence of HA 122 kDa (Polytech, Trieste, Italy) or HA >106 Da (Ostenil; Chemedica AG, München, Germany), the latter being indicated as high molecular weight (HMW) HA. Both HA compounds were checked for endotoxin content using LAL assay (detection level of 1 pg/ml), which was not found.
TNFα assay
TNFα produced by sputum cells and blood cells was measured by a modified two‐step sandwich type immunoassay (immunotrapping technique). The antibodies and standards were purchased from Biosource (Fleurus, Belgium). Cells were stimulated in apyrogen microwells (Nunc Maxisorp, VWR, Belgium) coated with anti‐TNFα antibodies (Ms X Hu TNFα clone 68B 6A3, Ms IgG1 and 68B 2B3, Ms IgG2) and, in parallel, recombinant TNFα standards were added to the wells. After 24 hours of incubation the wells were washed six times and incubated for 2 hours with a biotinylated anti‐TNFα antibody (Ms X Hu TNFα biotin clone 68B 3C5, Ms IgG1) followed by streptavidin‐horseradish peroxidase. The substrate chromogen solution TMB was used and absorbance was measured at 450 nm. Sensitivity was 10–15 pg/ml for the immunotrapping technique applied to the cell culture. The Biosource assay has been validated for conditioned medium but not for serum; this, together with improved sensitivity, led us to use the Quantikine high sensitivity R&D TNFα immunoassay (detection level 0.5 pg/ml) to measure TNFα in the serum samples. The Pelikine compact human TNFα assay (Sanquin, Amsterdam, the Netherlands) was used to detect TNFα in the sputum supernatant (detection limit 1 pg/ml).
IL‐8 assay
IL‐8 levels were determined using specific sandwich ELISA as described by Bouma and colleagues14 (detection limit 80 pg/ml).
Statistical analysis
The results are presented as median (range). Inter‐group comparisons were performed using the Kruskal‐Wallis H test; if differences were present (p<0.05), further analysis of subsets of groups were performed using the Mann‐Whitney U test or the Fisher's exact test (to compare categorical variables). To test the effect of LPS on TNFα production and to analyse the effect of HA on spontaneous and LPS induced TNFα release, intra‐groups comparisons were performed by the Friedman test and, if appropriate (at p<0.05), by a paired Wilcoxon rank sum test. Correlations between parameters were evaluated using Spearman's rank correlation analysis.
Statistical Package for Social Sciences (SPSS) Version 12.0 for Windows (SPSS Inc, Chicago, IL, USA) was used for statistical analysis, a p value of <0.05 denoting the presence of a significant statistical difference.
Results
Patient characteristics
The characteristics of the patients with COPD and the healthy control subjects are summarised in table 1. All four study groups consisted mostly of men and all COPD patients were current or ex‐smokers. In both control groups of non‐smokers and healthy smokers, all pulmonary function parameters were in the normal range which were significantly reduced in the COPD study groups. The FVC was low in the COPD patients, but this was explained by the importance of air trapping as reflected by high values of residual volume (RV) and total lung capacity (TLC). No differences in lung function were found between steroid treated and steroid free patients. Furthermore, all four study groups had comparable body mass index.
TNFα production in sputum cells
Sputum induction was performed successfully in the four study groups. Although COPD patients tended to have higher median total cell counts and neutrophil numbers together with reduced numbers of macrophage, the differences between the study groups were not significant (table 2). IL‐8 was significantly enhanced in the sputum of patients with COPD (table 2) and, when analysed in the whole group, correlated significantly with the neutrophil count (n = 36, r = 0.399, p = 0.021; data not shown). TNFα was detectable only in part of the sputum samples and levels of TNFα did not differ between study groups (table 2).
Table 2 Sputum characteristics.
| Control subjects | COPD patients | |||
|---|---|---|---|---|
| Non‐smoking | Smoking | Steroid free | Steroid treated | |
| (n = 9) | (n = 10) | (n = 9) | (n = 8) | |
| Sputum weight (g) | 4.3 (2.5–7.5) | 4.4 (1.0–7.4) | 3.1 (1.7–9.1) | 2.5 (1.4–4.9) |
| Squamous cells (%) | 18 (6–30) | 12.5 (3–54) | 19 (9–47) | 13 (0–45) |
| Viability (%) | 59 (27–79) | 61 (39–77) | 75 (25–87) | 74 (37–90) |
| 106 cells/g‡ | 0.5 (0.3–1.8) | 0.6 (0.1–2.4) | 0.9 (0.3–3.2) | 1.2 (0.5–11.7) |
| Neutrophils | ||||
| % | 52.0 (16.0–77.6) | 38.3 (4.6–87.0) | 57.6 (12.0–91.4) | 87.3 (5.2–95.0) |
| ×106/g | 0.29 (0.08–1.41) | 0.19 (0.02–0.81) | 0.47 (0.11–2.74) | 1.03 (0.02–10.67) |
| Macrophages | ||||
| % | 35.8 (14.0–58.4) | 35.4 (8.0–74.8) | 33.8 (6.6–77.0) | 8.3 (3.6–62.4) |
| ×106/g | 0.29 (0.07–0.65) | 0.35 (0.04–1.8) | 0.13 (0.06–0.87) | 0.23 (0.04–0.56) |
| Lymphocytes | ||||
| % | 2.8 (0.2–4.8) | 1.9 (02–10.0) | 1.4 (0.0–5.0) | 1.2 (0.0–4.6) |
| ×106/g | 0.01 (0.00–0.04) | 0.01 (0.00–0.24) | 0.01 (0.00–0.05) | 0.015 (0.00–0.12) |
| Eosinophils | ||||
| % | 0.0 (0.0–3.6) | 0.1 (0.0–6.2) | 0.0 (0.0–2.0) | 0.3 (0.0–49.6) |
| ×106/g | 0.0 (0.0–0.02) | 0.0 (0.0–0.09) | 0.0 (0.0–0.02) | 0.005 (0.0–0.35) |
| Epithelial cells | ||||
| % | 9.8 (0.8–28.2) | 10.2 (1.4–66.4) | 5.8 (1.0–39.4) | 1.1 (0.0–31.4) |
| ×106/g | 0.06 (0.0–0.17) | 0.11 (0.01–0.35) | 0.06 (0.02–0.83) | 0.035 (0.0–0.19) |
| IL‐8 positive samples | 5/9 | 8/10 | 9/9 | 8/8 |
| IL‐8 (ng/ml)‡ | 0.11 (0.08–2.81) | 0.42 (0.08–4.59) | 1.17 (0.28–25.0)* | 5.75 (0.91–14.40)*† |
| TNFα positive samples | 2/9 | 4/10 | 4/10 | 5/8 |
| TNFα (pg/ml)‡ | 1.0 (1.0–3.3) | 1.0 (1.0–8.9) | 1.0 (1.0–25.8) | 4.5 (1.0–307.8) |
Values are expressed as median (range).
Differences between groups were tested using the Kruskal‐Wallis H test and, if appropriate (p<0.05), subsequent analysis of subgroups was performed by the Mann‐Whitney U test.
*p<0.05 v non‐smoking controls; †p<0.05 v smoking controls.
‡Statistical differences between study groups were calculated based on all samples. In case of non‐detectable samples, the lower detection level of the assay was used (80 pg/ml for IL‐8, 1 pg/ml for TNFα).
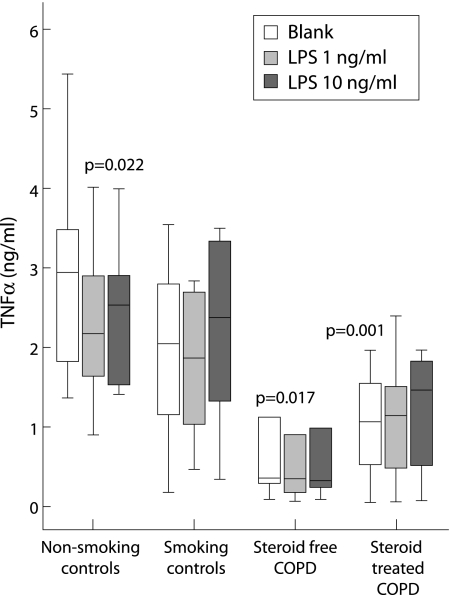
In order to analyse local TNFα production, sputum cells were cultured for 24 hours in the absence and presence of LPS, a bacterial cell wall constituent known to have strong inflammatory capacity. Sputum cells spontaneously produced considerable amounts of TNFα (fig 1). TNFα production by cells from COPD patients (both study groups) was significantly lower than for non‐smoking control subjects and tended to be different from smoking controls (steroid free COPD v smoking controls, p = 0.08; steroid treated COPD v smoking controls, p = 0.1). No relationship was seen between the viability of sputum cells and spontaneous TNFα production (n = 36, r = 0.124, p = 0.47). The presence of LPS (either 1 ng/ml or 10 ng/ml) did not affect spontaneous TNFα production for all groups, with the exception of LPS 1 ng/ml in the non‐smoking control subjects (p = 0.022, fig 1).
Figure 1 TNFα production by sputum cells of COPD patients and control subjects. TNFα production by sputum cells (20 000 cells/well) was measured by a dynamic immunoassay during a 24 hour culture period. Cells were incubated in RPMI 5% fetal calf serum without LPS (blank) or with LPS 1 ng/ml or 10 ng/ml. Data are presented as median with interquartile range (box) and range (whiskers) for 10 non‐smoking controls, 10 smoking controls, 9 steroid free COPD patients, and 8 steroid treated COPD patients. The p values indicate differences compared with spontaneous TNFα production by sputum cells from non‐smoking controls.
TNFα production in blood cells
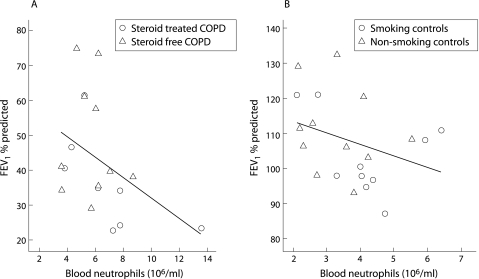
Significantly enhanced leucocyte numbers were present in the blood of both COPD subgroups compared with controls, which was mainly due to increased amounts of neutrophils (table 3). A negative correlation between blood neutrophil number and FEV1 % predicted was found for the whole study group (n = 39, r = −0.656, p<0.001) which was still present after analysis of patient and control subgroups (fig 2). Low levels of TNFα could be detected in the serum of all subjects, but no discrimination between study groups could be made based on the concentration of this cytokine (table 3).
Table 3 Blood differential cell count and TNFα level.
| Control subjects | COPD patients | |||
|---|---|---|---|---|
| Non‐smoking | Smoking | Steroid free | Steroid treated | |
| (n = 11) | (n = 10) | (n = 10) | (n = 8) | |
| Leucocytes (×106/ml) | 5.7 (4.4–8.7) | 7.2 (4.6–10.4) | 9.1 (7.1–13.9)*† | 8.7 (6.1–16.8)*† |
| Neutrophils | ||||
| % | 49.6 (44.4–73.0) | 57.1 (45.4–70.8) | 61.8 (49.9–68.4)* | 69.9 (54.3–85.5)*† |
| ×106/ml | 3.3 (2.13–5.5) | 4.1 (2.1–6.4) | 5.9 (3.6–8.7)* | 6.7 (3.7–13.6)*† |
| Lymphocytes | ||||
| % | 36.5 (17.8–42.7) | 32.6 (18.8–39.9) | 27.3 (19.8–39.9)* | 20.4 (9.1–33.7)*† |
| ×106/ml | 2.1 (1.3–3.5) | 2.0 (1.3–3.3) | 2.6 (1.5–3.7) | 1.8 (0.8–2.7) |
| Monocytes | ||||
| % | 5.7 (4.1–9.9) | 6.3 (3.5–8.7) | 6.4 (4.3–8.1) | 5.6 (3.3–8.7) |
| ×106/ml | 0.4 (0.3–0.7) | 0.4 (0.3–0.6) | 0.6 (0.3–1.1)*† | 0.5 (0.3–1.0) |
| Eosinophils | ||||
| % | 2.4 (0.9–4.2) | 1.7 (0.6–4.1) | 2.5 (0.8–7.6) | 1.7 (0.0–7.8) |
| ×106/ml | 0.1 (0.1–0.3) | 0.1 (0.1–0.3) | 0.2 (0.1–0.5) | 0.2 (0.0–0.9) |
| Basophils | ||||
| % | 0.7 (0.4–1.0) | 0.7 (0.4–1.3) | 0.6 (0.3–0.9) | 0.4 (0.0–1.2) |
| ×106/ml | 0.04 (0.02–0.06) | 0.05 (0.02–0.07) | 0.05 (0.03–0.1) | 0.03 (0.0–0.14) |
| TNFα (pg/ml) | 3.5 (1.7–9.6) | 3.4 (2.6–3.7) | 3.8 (2.5–6.1) | 3.6 (2.5–4.9) |
Values are expressed as median (range).
Differences between groups were tested using the Kruskal‐Wallis H test and, if appropriate (p<0.05), subsequent analysis of subgroups was performed by the Mann‐Whitney U test.
*p<0.05 v non‐smoking controls; †p<0.05 v smoking controls.
Figure 2 Relationship between number of neutrophils in the blood and FEV1 % predicted. A significant negative relationship was seen between the absolute number of neutrophils in peripheral blood and severity of airway obstruction as assessed by FEV1 % predicted in both (A) patients with COPD (n = 18, r = −0.489, p = 0.039) and (B) control subjects (n = 21, r = −0.438, p = 0.047).
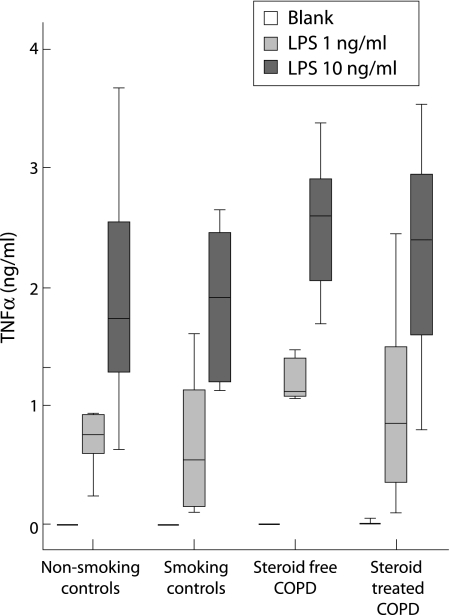
In contrast to sputum, culture of blood cells did not result in spontaneous production of TNFα. The release of TNFα was induced by LPS in a concentration dependent manner (fig 3). No differences were seen in LPS (1 ng and 10 ng/ml) induced TNFα release between study groups. Since TNFα release was measured in 1:20 diluted whole blood and leucocyte counts differed between groups, TNFα release per 20 000 leucocytes was calculated which did not result in differences between study subjects (data not shown).
Figure 3 TNFα production by blood cells of COPD patients and control subjects. Heparinised whole blood was diluted 1:20 in RPMI 5% fetal calf serum and TNFα production was measured by a dynamic immunoassay during a 24 hour culture period. Cells were incubated without LPS (blank) or with LPS 1 ng/ml or 10 ng/ml. Data are presented as median with interquartile range (box) and range (whiskers) for 11 non‐smoking controls, 10 smoking controls, 9 steroid free COPD patients, and 8 steroid treated COPD patients.
Effect of HA on TNFα production by sputum and blood cells
Since the extracellular matrix compound HA has been reported to modulate inflammatory reactions, the effect of HA on TNFα production by blood and sputum cells from patients and control subjects was analysed. Neither LMW HA (122 kDa) nor HMW HA (>103 kDa) had any effect on spontaneous TNFα production in blood (table 4), but both fragments partially blocked TNFα release by blood cells induced by LPS 1 ng/ml in all study groups (table 4). This inhibition was comparable for all three concentrations of HA tested (1 µg/ml, 10 μg/ml and 100 μg/ml; data not shown). The reduction in TNFα release by HA was not present when blood cells were stimulated with LPS 10 ng/ml, with the exception of the HMW HA fragments which lowered TNFα release in steroid free COPD patients (table 4). No constitutive effect of either HA fragment was observed on spontaneous or LPS induced TNFα release by sputum cells (data not shown).
Table 4 Effect of HA on spontaneous and LPS stimulated TNFα release in blood cells.
| Control subjects | COPD patients | ||||
|---|---|---|---|---|---|
| Non‐smoking | Smoking | Steroid free | Steroid treated | ||
| (n = 11) | (n = 10) | (n = 9) | (n = 8) | ||
| LPS 0 | None | <10 (<10–21) | <10 (<10–22) | <10 (<10–57) | <10 (<10–47) |
| HA 122 kDa | <10 (<10–19) | <10 (<10–14) | <10 (<10–27) | <10 (<10–25) | |
| HA >103 kDa | <10 (<10–15) | <10 (<10–29) | <10 (<10–37) | <10 (<10–105) | |
| LPS 1 ng/ml | None | 738 (125–3172) | 533 (98–1607) | 1117 (96–3057) | 829 (86–2468) |
| HA 122 kDa | 496 (16–1916)* | 189 (24–1547)* | 859 (74–2684)* | 404 (10–1433)* | |
| HA >103 kDa | 466 (10–2305)* | 122 (37–1784)* | 928 (117–2840)* | 558 (41–1783)* | |
| LPS 10 ng/ml | None | 1677 (625–3681) | 1902 (1110–2654) | 2619 (1694–3386) | 2405 (794–3543) |
| HA 122 kDa | 1501 (520–3513) | 1654 (540–2696) | 2534 (1151–3760) | 2296 (559–3080) | |
| HA >103 kDa | 1386 (421–3214) | 1708 (908–2606) | 2545 (1428–3225)† | 2477 (828–3264) | |
LPS, lipopolysaccharide; HA, hyaluronan.
Values are expressed as median (range).
TNFα was measured by ELISA and expressed in pg/ml. The detection level of the assay was 10 pg/ml, therefore samples that were not measurable are indicated as <10 pg/ml. The effect of 10 μg/ml HA 122 kDa and 10 μg/ml HA >103 kDa are shown. Intra‐group comparisons were performed by the Friedman test and, if appropriate (at p<0.05), by a paired Wilcoxon rank sum test.
*p<0.05 v TNFα production in response to LPS 1 ng/ml without HA added.
†p<0.05 v TNFα production in response to LPS 10 ng/ml without HA added.
Discussion
This study shows a clear difference between TNFα production in sputum cells and in blood cells in patients with COPD and control subjects. Sputum cells produced constitutively high levels of TNFα, which was significantly lower in COPD patients than in controls. LPS had no effect on sputum cell TNFα production. In contrast, blood cells produced no TNFα spontaneously, and there was a significant increase in TNFα production in response to LPS, which did not differ between the study groups. Further indications for discrepancies between local and systemic inflammation were obtained by the observation that the extracellular matrix compound HA partially blocked LPS induced TNFα release by blood cells but did not affect TNFα production by sputum cells. No indication for altered responsiveness of cells of COPD patients towards HA was obtained.
Steroids are known to have anti‐inflammatory effects ex vivo, but the effect of these drugs on COPD inflammation are not so clear. Inhaled steroids have been shown to reduce neutrophil cell counts in sputum,15 but other studies could not confirm this effect on airway inflammation.16 In the present study no differences in lung function and in local and systemic TNFα release were seen between subgroups of patients treated with inhaled steroids and steroid free patients, indicating that steroids may not have a dramatic effect on TNFα production ex vivo. Nevertheless, since this study is cross sectional, intervention studies are required to confirm this conclusion.
Sputum neutrophilia is generally accepted as a characteristic feature of COPD.6,17 Although there was a tendency in this study towards enhanced neutrophil numbers and a reduced macrophage count in patients, no significant differences in differential cell counts were seen between patients with COPD and control subjects. This could be due to the relatively small size of the study groups analysed. In addition, care has been taken to match control subjects with respect to age, since Thomas et al18 found that the induced sputum neutrophil count increased significantly with age. The relatively high neutrophil number observed in controls in this study compared with other studies6,17 may therefore be due to the older age of the controls, and could be the reason for the absence of significantly enhanced neutrophilia in the sputum of patients with COPD. However, raised sputum IL‐8 levels—another characteristic of COPD6,7—were found in our patient group and correlated with the neutrophil counts. Sputum TNFα was detectable only in part of the samples and did not discriminate between study groups; this is similar to previously published findings,7,19 although some authors also found raised TNFα levels in patients with COPD.6 This discrepancy could be due to differences in the severity of the COPD patients studied or to technical aspects such as the method of sputum processing or the TNFα assay used.
In order to study local TNFα production we used ex vivo sputum cell culture which we have previously shown is a suitable model for the study of airway cytokine production.20 High constitutive TNFα production by sputum cells was observed, which was significantly reduced in COPD patients compared with control subjects. The contrast between high TNFα production ex vivo and the low TNFα sputum levels observed in this study suggests either that the in vivo produced TNFα is immediately captured or consumed or, alternatively, that in vivo factors are present which modulate local TNFα production and are absent from in vitro cell cultures. Moreover, since this study was performed on a total cell population, further studies are needed to elucidate which sputum cells are the main producers of TNFα during culture ex vivo and to get more insight into the underlying mechanisms for the observed differences between COPD patients and control subjects.
Contrary to our findings, Profita et al21 showed enhanced TNFα production by sputum cells of patients with COPD. They found differences in the macrophage and neutrophil counts between patients and controls which could account for the discrepancy in the results. However, our data suggesting suppression of inflammatory potential in COPD are supported by the observation of reduced cytokine production by primary bronchial epithelial cells of COPD patients.22 The inflammatory response is critical to control the growth of pathogenic micro‐organisms, a process in which TNFα can play a central role by directly activating macrophages and neutrophils. Reduced production of TNFα by sputum cells could therefore contribute to impaired local defence and thus to enhanced bacterial colonisation, as seen in COPD.23 However, attention also has to be paid to the potential detrimental role of TNFα in COPD by its contribution to the destruction of lung parenchyma.24 Further studies are therefore required to determine the implication of reduced TNFα production by sputum cells ex vivo in COPD patients.
Increasing doses of LPS did not affect TNFα release by sputum cells, which suggests that these cells—in contrast to blood cells—are unresponsive to LPS. Reactivity of sputum cells to PHA and FMLP has been reported,25,26 indicating that this lack of response is specific for LPS. It is well known that macrophages exposed to suboptimal doses of LPS are rendered tolerant to subsequent exposure to LPS.27 We therefore speculate that continuous exposure to bacteria known to colonise airways has made cells tolerant to LPS. Moreover, the discrepancy in viability of sputum cells (which was approximately 60–70%) compared with blood cells previously shown to be above 95% (data not shown) could contribute to some of the observed differences and will be the subject of further study.
Significantly enhanced circulating leucocyte numbers were present in COPD patients, as reported previously,28 indicating a systemic inflammatory response. Moreover, a correlation was seen between the neutrophil count and FEV1. In this study no enhancement in circulating TNFα levels was detected in the COPD patients, all of whom had normal BMI. These data are therefore in line with other studies showing unaltered TNFα in the serum of patients of stable weight, whereas enhanced TNFα levels and TNFα production has been shown in patients losing weight.29,30 No difference in spontaneous and LPS induced TNFα release by whole blood was observed between the study groups. Similarly, Aldonyte et al31 reported no difference in basal and LPS stimulated TNFα release by monocytes of COPD patients compared with controls. However, in the latter study differences were detected in the production of IL‐8 and matrix metalloproteinase 9, indicating changes in production of a specific subset of mediators.
To date, the origin of the systemic inflammation, which is considered to have a potential pathogenic role in other systemic effects of COPD such as nutritional abnormalities and weight loss or skeletal muscle dysfunction,9 remains poorly understood. This study shows that TNFα production by sputum cells and by blood cells is regulated differently, suggesting that the inflammatory processes in the airways and the circulation are independent. This hypothesis is confirmed by studies showing no correlation between levels of inflammatory mediators in sputum and plasma.7,32 In addition, a recent short term study on the effect of the anti‐TNFα drug infliximab administered by infusion did not reveal beneficial effects on local inflammatory indices,33 further indicating that the local and systemic compartments have to be considered as two separate entities.
Studies on alveolar septal wall remodelling in mild to moderate emphysema show a loss of total tissue, interstitial thickening, and an increased number of interstitial fibroblasts and macrophages.34 One interesting compound of the extracellular matrix is HA, a pleiotropic glycosaminoglycan composed of repeating disaccharide units of N‐acetyl‐d‐glucosamine‐β (1→4)‐d‐glucuronic acid‐β (1→3). Accumulating evidence suggests that HA contributes to both homeostasis and disease.35 Enhanced circulating levels of HA have been reported in inflammatory disorders.11 Its involvement in the pathogenesis of COPD has been suggested by the observation of enhanced levels of HA in the bronchoalveolar lavage fluid and sputum of these patients.10,36 Evidence indicates that the biological effects of HA are dependent on its molecular weight, as LMW HA has been shown to be pro‐inflammatory and HMW HA to be anti‐inflammatory.12
In contrast to our expectations, the 122 kDa HA fragment used in this study did not induce TNFα release by blood or sputum cells. Stimulating effects of HA fragments of comparable size have been reported for macrophages,12 eosinophils,37 and renal epithelial cells.38 The difference in cell types analysed or the presence of endogenous proteins in the diluted whole blood, such as enzymes degrading HA or protein contaminants such as lysozyme which are able to bind HA, could account for this contradiction. Moreover, both the HMW HA and the 122 kDa HA fragment reduced the LPS induced TNFα release by blood cells. Since HA is reported to bind to the LPS receptor TLR4 on dendritic cells,39 we speculate that interaction of HA with TLR4 on blood cells did not lead to cellular activation but hindered LPS induced cellular activation. HA had no effect on sputum cell TNFα production. This could not be due to action of endogenous proteins which were washed away during isolation of cells. Our results did not provide an indication that cells derived from COPD patients showed altered sensitivity towards HA compared with control subjects.
Overall, this study suggests an independent regulation of local versus systemic inflammation in COPD. TNFα production by sputum cells seems to be impaired, which could result in defective local defence. Further studies are needed to elucidate these processes further. The extracellular matrix compound HA had an inhibitory effect on LPS induced TNFα release from blood cells which was not different between patients and controls.
Acknowledgements
The authors thank Prof Dr A Schols and Dr E C Creutzberg for expert assistance with the statistical evaluation of the data.
Abbreviations
COPD - chronic obstructive pulmonary disease
FEV1 - forced expiratory volume in 1 second
HA - hyaluronan
IL - interleukin
LPS - lipopolysaccharide
TNFα - tumour necrosis factor α
Footnotes
This study was supported by a grant from the Dutch Asthma Foundation.
Competing interests: EFMW serves as a consultant to GlaxoSmithKline (GSK) and is a member of scientific advisory boards for GSK, Boehringer Ingelheim, Astra Zeneca, Centocor and Numico and received lecture fees from GSK, Astra Zeneca, Boehringer Ingelheim, Pfizer and Numico. He received research grants between 2001 and 2004 from GSK, Astra Zeneca, Boehringer Ingelheim, Centocor and Numico.
References
- 1.Barnes P J, Shapiro S D, Pauwels R A. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 200322672–688. [DOI] [PubMed] [Google Scholar]
- 2.Di Stefano A, Capelli A, Lusuardi M.et al Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med 19981581277–1285. [DOI] [PubMed] [Google Scholar]
- 3.O'Shaughnessy T C, Ansari T W, Barnes N C.et al Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med 1997155852–857. [DOI] [PubMed] [Google Scholar]
- 4.Saetta M, Baraldo S, Corbino L.et al CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999160711–717. [DOI] [PubMed] [Google Scholar]
- 5.Hogg J C, Chu F, Utokaparch S.et al The nature of small‐airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 20043502645–2653. [DOI] [PubMed] [Google Scholar]
- 6.Keatings V M, Collins P D, Scott D M.et al Differences in interleukin‐8 and tumor necrosis factor‐alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996153530–534. [DOI] [PubMed] [Google Scholar]
- 7.Vernooy J H, Kucukaycan M, Jacobs J A.et al Local and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors are increased in sputum. Am J Respir Crit Care Med 20021661218–1224. [DOI] [PubMed] [Google Scholar]
- 8.Schols A M, Buurman W A, Staal van den Brekel A J.et al Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 199651819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agusti A G, Noguera A, Sauleda J.et al Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 200321347–360. [DOI] [PubMed] [Google Scholar]
- 10.Dentener M A, Vernooy J H, Hendriks S.et al Enhanced levels of hyaluronan in lungs of patients with COPD: relationship with lung function and local inflammation. Thorax 200560114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majeed M, McQueen F, Yeoman S.et al Relationship between serum hyaluronic acid level and disease activity in early rheumatoid arthritis. Ann Rheum Dis 2004631166–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKee C M, Penno M B, Cowman M.et al Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest 1996982403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delvaux M, Henket M, Lau L.et al Nebulised salbutamol administered during sputum induction improves bronchoprotection in patients with asthma. Thorax 200459111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouma M G, Stad R K, van den Wildenberg F A.et al Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol 19941534159–4168. [PubMed] [Google Scholar]
- 15.Confalonieri M, Mainardi E, Della Porta R.et al Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary disease. Thorax 199853583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culpitt S V, Maziak W, Loukidis S.et al Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 19991601635–1639. [DOI] [PubMed] [Google Scholar]
- 17.Ronchi M C, Piragino C, Rosi E.et al Role of sputum differential cell count in detecting airway inflammation in patients with chronic bronchial asthma or COPD. Thorax 1996511000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas R A, Green R H, Brightling C E.et al The influence of age on induced sputum differential cell counts in normal subjects. Chest 20041261811–1814. [DOI] [PubMed] [Google Scholar]
- 19.Drost E M, Skwarski K M, Sauleda J.et al Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 200560293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bettiol J, Sele J, Henket M.et al Cytokine production from sputum cells after allergenic challenge in IgE‐mediated asthma. Allergy 2002571145–1150. [DOI] [PubMed] [Google Scholar]
- 21.Profita M, Chiappara G, Mirabella F.et al Effect of cilomilast (Ariflo) on TNF‐alpha, IL‐8, and GM‐CSF release by airway cells of patients with COPD. Thorax 200358573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel I S, Roberts N J, Lloyd‐Owen S J.et al Airway epithelial inflammatory responses and clinical parameters in COPD. Eur Respir J 20032294–99. [DOI] [PubMed] [Google Scholar]
- 23.Cabello H, Torres A, Celis R.et al Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J 1997101137–1144. [DOI] [PubMed] [Google Scholar]
- 24.Churg A, Wang R D, Tai H.et al Tumor necrosis factor‐alpha drives 70% of cigarette smoke‐induced emphysema in the mouse. Am J Respir Crit Care Med 2004170492–498. [DOI] [PubMed] [Google Scholar]
- 25.Liu L Y, Swensen C A, Kelly E A.et al The relationship of sputum eosinophilia and sputum cell generation of IL‐5. J Allergy Clin Immunol 20001061063–1069. [DOI] [PubMed] [Google Scholar]
- 26.Beeh K M, Beier J, Lerch C.et al Effects of piclamilast, a selective phosphodiesterase‐4 inhibitor, on oxidative burst of sputum cells from mild asthmatics and stable COPD patients. Lung 2004182369–377. [DOI] [PubMed] [Google Scholar]
- 27.Fujihara M, Muroi M, Tanamoto K.et al Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther 2003100171–194. [DOI] [PubMed] [Google Scholar]
- 28.Gan W Q, Man S F, Senthilselvan A.et al Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta‐analysis. Thorax 200459574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Godoy I, Donahoe M, Calhoun W J.et al Elevated TNF‐alpha production by peripheral blood monocytes of weight‐losing COPD patients. Am J Respir Crit Care Med 1996153633–637. [DOI] [PubMed] [Google Scholar]
- 30.Di Francia M, Barbier D, Mege J L.et al Tumor necrosis factor‐alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 19941501453–1455. [DOI] [PubMed] [Google Scholar]
- 31.Aldonyte R, Jansson L, Piitulainen E.et al Circulating monocytes from healthy individuals and COPD patients. Respir Res 2003411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurst J R, Wilkinson T M, Perera W R.et al Relationships among bacteria, upper airway, lower airway, and systemic inflammation in COPD. Chest 20051271219–1226. [DOI] [PubMed] [Google Scholar]
- 33.van der Vaart H, Koeter G H, Postma D S.et al First study of infliximab treatment in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005172465–469. [DOI] [PubMed] [Google Scholar]
- 34.Vlahovic G, Russell M L, Mercer R R.et al Cellular and connective tissue changes in alveolar septal walls in emphysema. Am J Respir Crit Care Med 19991602086–2092. [DOI] [PubMed] [Google Scholar]
- 35.McDonald J, Hascall V C. Hyaluronan minireview series. J Biol Chem 20022774575–4579. [DOI] [PubMed] [Google Scholar]
- 36.Song W D, Zhang A C, Pang Y Y.et al Fibronectin and hyaluronan in bronchoalveolar lavage fluid from young patients with chronic obstructive pulmonary diseases. Respiration 199562125–129. [DOI] [PubMed] [Google Scholar]
- 37.Ohkawara Y, Tamura G, Iwasaki T.et al Activation and transforming growth factor‐beta production in eosinophils by hyaluronan. Am J Respir Cell Mol Biol 200023444–451. [DOI] [PubMed] [Google Scholar]
- 38.Oertli B, Beck‐Schimmer B, Fan X.et al Mechanisms of hyaluronan‐induced up‐regulation of ICAM‐1 and VCAM‐1 expression by murine kidney tubular epithelial cells: hyaluronan triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor‐kappa B and activating protein‐1. J Immunol 19981613431–3437. [PubMed] [Google Scholar]
- 39.Termeer C, Benedix F, Sleeman J.et al Oligosaccharides of hyaluronan activate dendritic cells via toll‐like receptor 4. J Exp Med 200219599–111. [DOI] [PMC free article] [PubMed] [Google Scholar]