Abstract
Background
Prognosis in chronic obstructive pulmonary disease (COPD) is poorly predicted by indices of air flow obstruction, because other factors that reflect the systemic nature of the disease also influence prognosis.
Objective
To test the hypothesis that a reduction in quadriceps maximal voluntary contraction force (QMVC) is a useful predictor of mortality in patients with COPD.
Methods
A mortality questionnaire was sent to the primary care physician of 184 patients with COPD who had undergone quadriceps strength measurement over the past 5 years. QMVC was expressed as a percentage of the patient's body mass index. The end point measured was death or lung transplantation, and median (range) follow‐up was 38 (1–54) months.
Results
Data were obtained for 162 patients (108 men and 54 women) with a mean (SD) percentage of forced expiratory volume in 1 s (FEV1) predicted of 35.6 (16.2), giving a response rate of 88%. Transplant‐free survival of the cohort was 93.5% at 1 year and 87.1% at 2 years. Cox regression models showed that the mortality risk increased with increasing age and with reducing QMVC. Only age (HR 1.72 (95% CI 1.14 to 2.6); p = 0.01) and QMVC (HR 0.91 (95% CI 0.83 to 0.99); p = 0.036) continued to be significant predictors of mortality when controlled for other variables in the multivariate analysis.
Conclusion
QMVC is simple and provides more powerful prognostic information on COPD than that provided by age, body mass index and forced expiratory volume in 1 s.
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the world,1 but prognosis is only poorly predicted by indices of air flow obstruction. Given this limitation, a new severity classification, the Body Mass Index, Airflow Obstruction, Dyspnoea and Exercise Capacity (BODE) Index,2 has been proposed that takes into account the multicomponent nature of COPD with considerable emphasis being placed on the body mass index (BMI) as an indicator of poor prognosis.3,4,5,6 However, many investigators consider that it is, more specifically, the loss of skeletal muscle mass which confers a poorer prognosis in patients with COPD.7,8,9,10 Muscle mass depletion is associated with reduced exercise performance,11,12 increased dyspnoea13 and worse health‐related quality of life.14 Similarly, skeletal muscle weakness is a common finding in COPD and is associated with reduced exercise capacity.15,16,17 As exercise capacity is thought to be an important factor in determining mortality in COPD,18 it perhaps follows that muscle weakness should also predict mortality.
In a recent paper by Marquis et al,7 CT scanning was used to measure the mid‐thigh cross‐sectional area (MTCSACT) in patients with COPD. This radiological measure of quadriceps bulk was shown to predict mortality better than BMI, and this was particularly so in patients with more severe COPD (forced expiratory volume in 1 s, (FEV1) <50%). This measure is attractive as a single reproducible predictor of mortality from COPD, but some obvious drawbacks limit its more widespread use. In particular the use of CT scanning has resource implications and involves a considerable exposure to radiation. We therefore hypothesised that a simple functional measure of strength, the quadriceps maximum voluntary contraction force (QMVC), might also predict mortality in patients with COPD.
Methods
The study cohort comprised 184 patients with COPD, recruited without further selection from outpatient clinics at the Royal Brompton Hospital (London, UK), a tertiary referral centre, and King's College Hospital (London, UK), in whom quadriceps strength measurements had been performed in the preceding 5 years. All patients underwent baseline anthropometric measurements including bioelectrical impedance analysis and pulmonary function tests, as well as quadriceps strength measurement. The primary end point was time to death or lung transplantation (termed transplant‐free survival, TFS), calculated from the date when quadriceps strength was measured until the reference date of 20 July 2005, when the analysis was performed. The status of the patient (alive, dead or lung transplant recipient) was ascertained by a questionnaire sent to the patient's primary care physician. We also inquired about exacerbations of COPD that required hospital admission, smoking status, comorbidities, drug treatments and whether they had undergone a course of pulmonary rehabilitation. Patients were excluded from this study if they had considerable comorbidity. The Royal Brompton Hospital Research Ethics Committee approved the study.
Pulmonary function testing
All patients had COPD diagnosed according to international guidelines.19 Pulmonary function testing was performed by standardised techniques in the lung function departments of the Royal Brompton and King's College Hospitals. Patients were subdivided into Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages on the basis of their FEV1.
Fat‐free mass measurements
Fat‐free mass (FFM) was determined using bioelectrical impedance analysis (Bodystat 1500, Bodystat, Isle Of Man, UK) and a disease‐specific regression equation.20
Quadriceps Measurements
QMVC was measured using the technique of Edwards et al.21 Patients sat in a purpose‐built chair with an inextensible strap connecting the ankle of their dominant leg to a strain gauge (Strainstall, Cowes, UK). The force signal was amplified and passed to a computer running LabView version 4.1 software (National Instruments, Austin, Texas). The linearity of the strain gauge is factory certified from 0 to 100 kg. The equipment was calibrated using a suspended weight before testing each patient. Care was taken to ensure that patients' knees were flexed to 90°, and that all the strain gauge and couplings were all aligned to ensure that the contraction was isometric. Patients performed at least three sustained maximal isometric quadriceps contractions of between 5 and 10 s duration. The force produced was visible online to both the patient and investigator to allow positive feedback, and vigorous encouragement was given. There was a gap of 30–60 s between each contraction to allow time to recover from each effort. The best QMVC was then expressed as a function of the patient's BMI.
Statistical analysis
TFS distribution was estimated using the Kaplan–Meier product moment estimator. A univariate analysis, based on the Cox proportional hazards model, where survival status at 20 July 2005 was used as the dependent variable, was performed to identify prognostic factors for TFS. Variables considered were age (divided into 10‐year groups), BMI, Fat‐Free Mass Index (FFMI), FEV1 (percentage predicted) and QMVC (as a percentage of BMI). A multivariate Cox model was then fitted, in which all factors were considered. A stepwise backward variable selection procedure was implemented to remove the non‐significant variables from the multiple models. The validity of the proportional hazards assumptions was assessed using the Grambsch and Therneau goodness‐of‐fit test22 and the validity of the log‐linearity assumptions by fitting generalised additive models with splines to the residuals. Additionally, the discriminative ability of different multiple models was compared using the D measure of Royston and Sauerbrei,23 as well as the D‐based version of Kent and O'Quigley's24 measure of dependence (R2 measure). Data are expressed as mean (SD) values unless otherwise stated.
Results
The response rate to the questionnaire was 88%, and therefore the data expressed in this paper are for 162 patients (108 men and 54 women). Table 1 shows the patient demographics, and there were no significant differences between the patients in whom we obtained outcome data and those in whom we did not. During follow‐up, 36 deaths occurred, as well as 2 lung transplants, and 31 patients had at least 1 hospital admission owing to an exacerbation of COPD (3 followed by death and 1 by a lung transplant).
Table 1 Demographics of the patients.
| Complete patient data | No outcome data | |
|---|---|---|
| n = 162 (108 men, 54 women) | n = 22 (12 men, 10 women) | |
| Mean (SD) | Mean (SD) | |
| Age when tested (years) | 63.7 (9.3) | 66.7 (9.5) |
| Height (m) | 1.7 (0.09) | 1.67 (0.09) |
| Weight (kg) | 70.2 (16.3) | 70.4 (15.7) |
| BMI (kg/m2) | 24.4 (5) | 25.2 (4.7) |
| FFMI in men (kg/m2) | 17.1 (2.3) | 17.9 (2.5) |
| FFMI in women (kg/m2) | 15.8 (2.3) | 15.7 (1.7) |
| FEV1(l) | 0.98 (0.44) | 1.02 (0.3) |
| FEV1% predicted | 35.6 (16.2) | 42.2 (9.3) |
| DLCO % predicted | 37.3 (17.6) | 41.2 (12.1) |
| GOLD stage | ||
| 0 | 0 (0%) | 0 (0%) |
| 1 | 2 (1.2%) | 0 (0%) |
| 2 | 29 (17.9%) | 3 (13.6%) |
| 3 | 56 (34.6%) | 9 (40.9%) |
| 4 | 75 (46.3%) | 10 (45.5%) |
| QMVC (kg) | 32.4 (11.5) | 35.7 (13.3) |
| QMVC as a % of BMI | 135.5 (46.7) | 142.7 (50.5) |
BMI, body mass index; DLCO: diffusing capacity of lungs for carbon monoxide; FEV1: forced expiratory volume in one second; FFMI, Fat‐Free Mass Index; GOLD, Global Initiative for Chronic Obstructive Lung Disease; QMVC, quadriceps maximal voluntary contraction.
We found no significant differences between the patients in whom outcome data were available and those for whom they were not.
All patients received standard treatment for COPD, with 20 patients taking long‐term oral steroids at the time of quadriceps measurement. Nineteen patients were continuing smokers at the time of measurement. Seventy four patients had completed or subsequently completed a course of pulmonary rehabilitation. Patients with serious comorbidity were excluded from the analysis (ie, their primary care physician was not sent a questionnaire), and of the remaining cohort, 11 patients had treated hypertension, eight had mild ischaemic heart disease and one had type 2 diabetes mellitus.
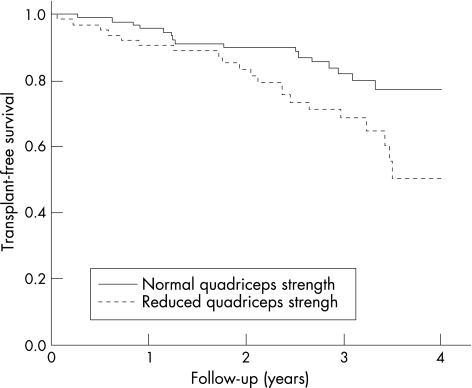
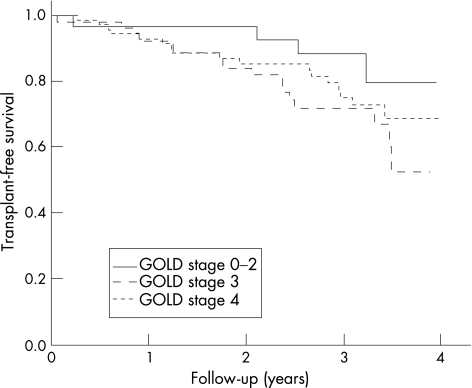
Most of the patients had moderate to severe COPD that reflects the type of patients seen in hospital outpatient clinics in the UK. Figure 1 shows the TFS curve of the cohort. As only two patients were followed up for >4 years, one of whom died, the data were censored at 4 years. The median length of follow‐up was 38 months, with a range of 1–54 months. The TFS of the cohort was 93.5% at 1 year and 87.1% at 2 years. The TFS curves for patients with normal and reduced quadriceps strength (QMVC ⩾120% or <120% of BMI, respectively) were significantly different (p = 0.017; fig 2). The TFS separated by the GOLD stage shows that mortality increases with worsening lung function (fig 3). Although the curves show the expected trend, they do not reach significance (stages 0–2 v 3–4; p = 0.17).
Figure 1 Transplant‐free survival for the whole chronic obstructive pulmonary disese cohort (solid curve) with 95% CI (dashed line). Censoring times are represented by crosses (+).
Figure 2 Transplant‐free survival for patients with normal and reduced quadriceps strength, as defined by a quadriceps maximal voluntary contraction force >120% or <120% of body mass index. The curves are significantly different, p = 0.017.
Figure 3 Transplant‐free survival separated by Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage. The curves were not significantly different (stages 0–2 v 3–4; p = 0.17).
Table 2 shows the results of univariate and multivariate Cox models. The univariate analysis shows that the mortality risk increased with increasing age and with reducing QMVC. Among all factors in the multivariate analysis, only age and QMVC wielded a significant association with survival. Diffusing capacity of the lungs for carbon monoxide was not considered in the multivariate analysis as data from 23 patients were missing. Being treated with oral steroids is a strong predictor of mortality: hazard ratio (HR) 3.68 (95% confidence interval (CI) 1.84 to 7.34); p = 0.001. No significant association was found between an acute exacerbation and the hazard of death or transplantation (HR 0.65, 95% CI 0.23 to 1.88; p = 0.43). In fact, only 3 of the 32 patients who had exacerbations died, compared with 32 of the 130 who did not. When compared with a model including age, sex, BMI and FEV1 (% predicted), the selected model using only QMVC, sex and age yielded an explained variation (R2) of 0.29 v 0.26, indicating improved prognostic power. The D measure of prognostic discrimination for these models was 1.02 v 0.93, in agreement with the previous result.
Table 2 Assessment of prognostic factors using Cox proportional hazard models.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Men | 1.3 (0.64.to 2.62) | 0.47 | 1.61 (0.71 to 3.64) | 0.25 |
| Age (per 10 years) | 1.68 (1.19 to 2.36) | 0.003 | 1.72 (1.14 to 2.6) | 0.01 |
| BMI (kg/m2) | 0.96 (0.9 to 1.03) | 0.3 | 0.91 (0.8 to 1.05) | 0.21 |
| FFMI (kg/m2) | 0.96 (0.82 to 1.11) | 0.58 | 1.08 (0.81 to 1.45) | 0.6 |
| FEV1 (% predicted) | 0.99 (0.97 to 1.01) | 0.17 | 0.98 (0.96 to 1.01) | 0.13 |
| DLCO (%) | 0.97 (0.95 to 0.99) | 0.016 | ||
| QMVC (10% of BMI) | 0.92 (0.85 to 0.99) | 0.022 | 0.91 (0.83 to 0.99) | 0.036 |
| Currently smoking | 1.79 (0.82 to 3.93) | 0.14 | 2.06 (0.85 to 5) | 0.11 |
| Pulmonary rehabilitation | 0.95 (0.5 to 1.8) | 0.88 | 1.57 (0.75 to 3.3) | 0.23 |
BMI, body mass index; DLCO, diffusing capacity of lungs for carbon monoxide; FEV1, forced expiratory volume in 1 s; FFMI, Fat‐Free Mass Index; QMVC, quadriceps maximal voluntary contraction.
DLCO was not included in the multivariate analysis as data on 23 patients were missing.
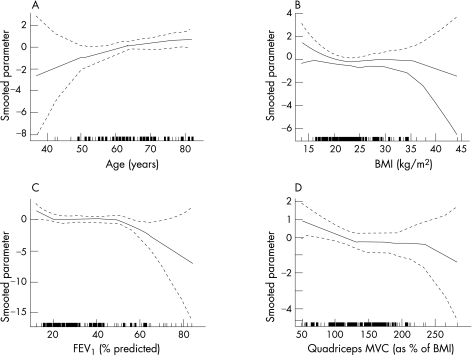
A review of the HRs for individual variables (fig 4) did not challenge the linearity hypotheses of the Cox models, nor Grambsch and Therneau's goodness‐of‐fit test (all p<0.25). The relationship between the risk of death and increasing age and reducing QMVC seems roughly linear, whereas the relationship between BMI and FEV1 and the risk of death seemed, to visual inspection, flatter when considering a BMI between 20 and 30 kg/m2 or an FEV1 <50% of predicted, suggesting poorer prognostic power in these ranges for these variables.
Figure 4 Test of functional form for (A) age, (B) body mass index (BMI), (C) forced expiratory volume in 1 s (FEV1) percentage predicted and (D) quadriceps maximal voluntary contraction (QMVC) data. The y axis shows the spline estimates of log HRs for death (solid lines) with 95% CI (dashed lines) for each variable. The small lines on the x axis show where individual patients lie within the range.
Discussion
We found that, in patients with COPD, quadriceps muscle strength adds prognostic information to that provided by age, BMI and FEV1. We believe that, in common with MTCSACT, QMVC reflects quadriceps muscle bulk and can be indicative of skeletal muscle dysfunction, but this physiological measurement is cheap and radiation free and may have greater functional relevance. Measurement of quadriceps strength could be used to identify high‐risk patients with COPD who have peripheral muscle weakness and who may obtain greater benefit from rehabilitation or nutritional supplementation.
Critique of the method
Our questionnaire did not specify the cause of death so the data reflect all‐cause mortality. The mortality in our study, an annual death rate of approximately 7%, is similar to other studies on comparable patients, ranging from 5% in the study by Marquis et al7 to 9% in the study by Slinde et al8 We had a response rate of 88% from the questionnaires, and this was partly because of some patients no longer being registered with their original primary care physician. However, we doubt that the incompleteness of the data jeopardises our conclusions as both groups were similar with respect to baseline characteristics. We also acknowledge that as the Brompton Hospital acts as a tertiary referral centre, our patients may not be wholly typical of the generality of patients with COPD; our hypothesis should be retested in a primary care setting.
A further limitation of the study is that we measured only whole‐body FFM rather than regional muscle bulk. Thus, we were unable to identify whether the poorer prognosis conferred by weakness is specifically due to a reduction in quadriceps bulk or a reduction in quadriceps specific force. Although this could be an interesting area for future study, it does not detract from the finding that QMVC has strong prognostic power.
Quadriceps strength in normal human beings bears a reasonably close relationship with total body weight, and Edwards et al21 recommended that quadriceps strength be normalised against body weight. This approach relied on an unstated assumption that BMI did not vary greatly within his healthy population, and therefore that quadriceps muscle strength enjoyed a reasonably constant relationship with body weight (as a surrogate for height); in fact, Edwards et al21 reported neither the height nor BMI of their subjects. In patients with severe COPD, selective loss of quadriceps muscle bulk occurs, and as we hypothesised that it was this mechanism that confers the poor prognosis, it seemed inappropriate to discount the effect of height (and by inference femur length) on QMVC. For this reason, we expressed QMVC as a function of BMI. In addition, we performed analyses expressing QMVC as a function of body weight or FFM and this did not materially alter our conclusions, although the predictive power was slightly diminished with the body weight and increased with FFM.
QMVC is a non‐invasive test, is simple to perform and gives a functional assessment of muscle bulk. Although we could additionally have performed magnetic stimulation of the femoral nerve, using the rationale that volitional measures are open to the criticism that weakness is due to reduced motivation or aptitude,25 we did not do this in most patients. The fact that QMVC emerged as a powerful prognostic indicator suggests that these reservations do not detract from the value of the measurement.
Exercise capacity or dyspnoea scores were not available for these patients; therefore, we were unable to calculate our cohort's BODE Index and could not compare our predictive model with that of Celli et al.2 However, exercise capacity is associated with quadriceps strength and the data show that our model, which includes only age, sex and quadriceps strength, is a useful predictor of mortality and is easier to perform. In the BODE grading system, the four variables that had the strongest association with 1‐year mortality (BMI, FEV1, modified Medical Research Council dyspnoea score and 6‐min walk distances) had a generalised R2 = 0.21, whereas in our study, a model including age, sex and QMVC yielded an explained variation over the time course of the study of R2 = 0.29. Although direct comparison of the prognostic value of these two models is difficult as we are unable to compute the same data, the power of our model is at least of a similar order of magnitude.
Importance of the findings
In the general population, quadriceps weakness has been shown to be a predictor of mortality.26 Hence, our results are consistent with the hypothesis that factors that contribute to quadriceps weakness, such as inactivity, may also lead to reduced survival. However, the magnitude of the weakness observed in our patients with moderate to severe COPD is more marked than that observed in the normal population. We have previously shown that patients with COPD had a mean QMVC of 34.4 kg compared with 43.8 kg in the healthy, age‐matched controls 27; therefore we do not think our data simply reflect ageing.
Our findings suggest that quadriceps strength is a better marker at predicting mortality than either BMI or FFMI. In Marquis et al's study7 the subjects' mean BMI (26 kg/m2) was in the normal range, yet the MTCSACT values were 72% of the normal, which indicates that quadriceps muscle mass rather than body weight is the important physiological variable predicting survival. This suggests that the loss of muscle mass has more ominous implications for prognosis than the loss of other body compartments. Consistent with these data we did not find that FFMI was predictive of mortality, which is in contrast with other studies,8,10 although absolute values for FFMI are not greatly different between these studies. The discordance between changes in FFM and quadriceps strength seems to support the importance of local rather than systemic factors in producing weakness.
In COPD, systemic factors such as inflammation, hypoxia and nutritional depletion may interact with local factors such as muscle activity level or perfusion to produce muscle weakness. The question remains as to the extent to which muscle weakness is a generalised, systemically determined phenomenon or one that predominantly affects the lower limbs as a result of disuse. The “compartment theory” is based on the premise that changes in muscle function depend on the demands placed on the muscle in question and is supported by the observation that different changes are found in different muscle groups.28 Thus, it is argued that patients walk less because of dyspnoea, which leads to disuse atrophy and quadriceps weakness. Upper limb strength is relatively maintained in patients with COPD, because there is a preservation of upper body activity and the shoulder girdle muscles are accessory muscles of respiration.17 Adductor pollicis twitch force is normal whereas quadriceps twitch is reduced in patients with COPD.29 Therefore, it is interesting that the mid‐arm circumference, which reflects upper limb muscle bulk, has been shown to predict mortality.9 We have also recently shown that the expiratory muscles (which are active in patients with COPD) have at least normal strength in a cohort of patients with COPD.30 We therefore believe that the predominance of published data is in favour of the characteristic abnormality in COPD being an isolated locomotor muscle weakness.
The prognostic value of QMVC does not establish a causal relationship. Our study suggests that reduced quadriceps strength predicts mortality; however, it does not help in elucidating the reasons for this decline or when this begins in patients with COPD. We are not sure whether the natural history of quadriceps weakness is a slow deterioration, with disease progression or reduced activity, or, as suggested by the data of Spruit et al31 a stepwise decline associated with exacerbations. Only 17% of our cohort had an exacerbation requiring hospital admission during follow‐up, indicating that the majority were not frequent exacerbators, despite most of the patients having quadriceps weakness. This suggests that serious exacerbations may not be necessary to cause quadriceps muscle deterioration. It is possible that quadriceps weakness is a surrogate marker for patients' reduced generalised performance status, but it may also be a systemic manifestation of generalised inflammation in COPD. It has been shown that reduced physical activity in itself is an independent risk factor for hospital admission.32 We do not know the effect of improving muscle strength on mortality, although our group has shown that early pulmonary rehabilitation after hospitalisation does improve quality of life and exercise capacity, and reduces further hospital attendances.33
In conclusion, quadriceps strength measured by a maximal voluntary isometric contraction is useful in predicting the mortality of patients with COPD. This easily performed measurement could be widely performed in the lung function laboratory and could serve to identify patients at higher risk of death.
Abbreviations
BMI - body mass index
BODE - Body Mass Index, Airflow Obstruction, Dyspnoea and Exercise Capacity
COPD - chronic obstructive pulmonary disease
MTCSACT - mid‐thigh cross‐sectional area measured by CT scanning
FEV1 - forced expiratory volume in 1 s
FFM - fat free mass
FFMI - Fat Free Mass Index
GOLD - Global Initiative for Chronic Obstructive Lung Disease
QMVC - quadriceps maximum voluntary contraction force
TFS - transplant‐free survival
Footnotes
Competing interests: None.
References
- 1.World Health Organization World Health Report. Geneva: WHO, 2000
- 2.Celli B R, Cote C G, Marin J M.et al The Body‐Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index in chronic obstructive pulmonary disease. N Engl J Med 20043501005–1012. [DOI] [PubMed] [Google Scholar]
- 3.Schols A M W J, Slangen J, Volovics L.et al Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 19981571791–1797. [DOI] [PubMed] [Google Scholar]
- 4.Landbo C, Prescott E, Lange P.et al Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 19991601856–1861. [DOI] [PubMed] [Google Scholar]
- 5.Prescott E, Almdal T, Mikkelsen K L.et al Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J 200220539–544. [DOI] [PubMed] [Google Scholar]
- 6.Chailleux E, Laaban J ‐ P, Veale D. Prognostic value of nutritional depletion in patients with COPD treated by long‐term oxygen therapy: data from the ANTADIR observatory. Chest 20031231460–1466. [DOI] [PubMed] [Google Scholar]
- 7.Marquis K, Debigare R, Lacasse Y.et al Midthigh muscle cross‐sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002166809–813. [DOI] [PubMed] [Google Scholar]
- 8.Slinde F, Gronberg A, Engstrom C ‐ P.et al Body composition by bioelectrical impedance predicts mortality in chronic obstructive pulmonary disease patients. Respir Med 2005991004–1009. [DOI] [PubMed] [Google Scholar]
- 9.Soler‐Cataluna J J, Sanchez‐Sanchez L, Martinez‐Garcia M A.et al Mid‐arm muscle area is a better predictor of mortality than body mass index in COPD. Chest 20051282108–2115. [DOI] [PubMed] [Google Scholar]
- 10.Schols A M, Broekhuizen R, Weling‐Scheepers C A.et al Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 20058253–59. [DOI] [PubMed] [Google Scholar]
- 11.Baarends E, Schols A, Mostert R.et al Peak exercise response in relation to tissue depletion in patients with chronic obstructive pulmonary disease. Eur Respir J 1997102807–2813. [DOI] [PubMed] [Google Scholar]
- 12.Schols A M, Mostert R, Soeters P B.et al Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax 199146695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahebjami H, Sathianpitayakul E. Influence of body weight on the severity of dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000161886–890. [DOI] [PubMed] [Google Scholar]
- 14.Mostert R, Goris A, Weling‐Scheepers C.et al Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med 200094859–867. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton A L, Killian K J, Summers E.et al Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med 1995152(Pt 1)2021–2031. [DOI] [PubMed] [Google Scholar]
- 16.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996153976–980. [DOI] [PubMed] [Google Scholar]
- 17.Bernard S, LeBlanc P, Whittom F.et al Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998158629–634. [DOI] [PubMed] [Google Scholar]
- 18.Oga T, Nishimura K, Tsukino M.et al Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003167544–549. [DOI] [PubMed] [Google Scholar]
- 19.Pauwels R A, Buist A S, Calverley P M.et al Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 20011631256–1276. [DOI] [PubMed] [Google Scholar]
- 20.Steiner M C, Barton R L, Singh S J.et al Bedside methods versus dual energy x rayabsorptiometry for body composition measurement in COPD. Eur Respir J 200219626–631. [DOI] [PubMed] [Google Scholar]
- 21.Edwards R H, Young A, Hosking G P.et al Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med 197752283–290. [DOI] [PubMed] [Google Scholar]
- 22.Grambsch P M, Therneau T M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 199481515–526. [Google Scholar]
- 23.Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med 200423723–748. [DOI] [PubMed] [Google Scholar]
- 24.Kent J T, O'Quigley J. Measures of dependence for censored survival data. Biometrika 198875525–534. [Google Scholar]
- 25.Man W D, Moxham J, Polkey M I. Magnetic stimulation for the measurement of respiratory and skeletal muscle function. Eur Respir J 200424846–860. [DOI] [PubMed] [Google Scholar]
- 26.Newman A B, Kupelian V, Visser M.et al Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol 20066172–77. [DOI] [PubMed] [Google Scholar]
- 27.Hopkinson N S, Nickol A H, Payne J.et al Angiotensin converting enzyme genotype and strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004170395–399. [DOI] [PubMed] [Google Scholar]
- 28.Gea J G, Pasto M, Carmona M A.et al Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur Respir J 200117939–945. [DOI] [PubMed] [Google Scholar]
- 29.Man W D, Soliman M G, Nikoletou D.et al Non‐volitional assessment of skeletal muscle strength in patients with chronic obstructive pulmonary disease. Thorax 200358665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Man W D ‐ C, Hopkinson N S, Harraf F.et al Abdominal muscle and quadriceps strength in chronic obstructive pulmonary disease. Thorax 200560718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spruit M A, Gosselink R, Troosters T.et al Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF‐I. Thorax 200358752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia‐Aymerich J, Farrero E, Felez M A.et al Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 200358100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Man W D ‐ C, Polkey M I, Donaldson N.et al Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 20043291209. [DOI] [PMC free article] [PubMed] [Google Scholar]