Abstract
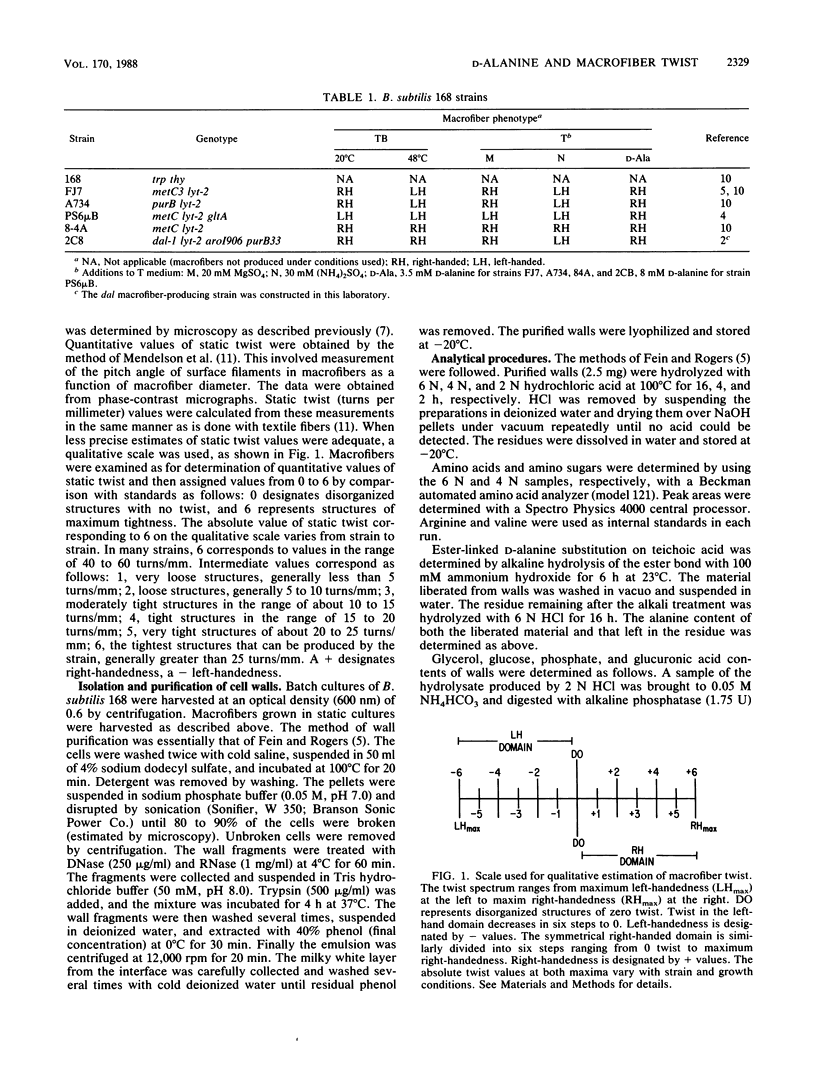
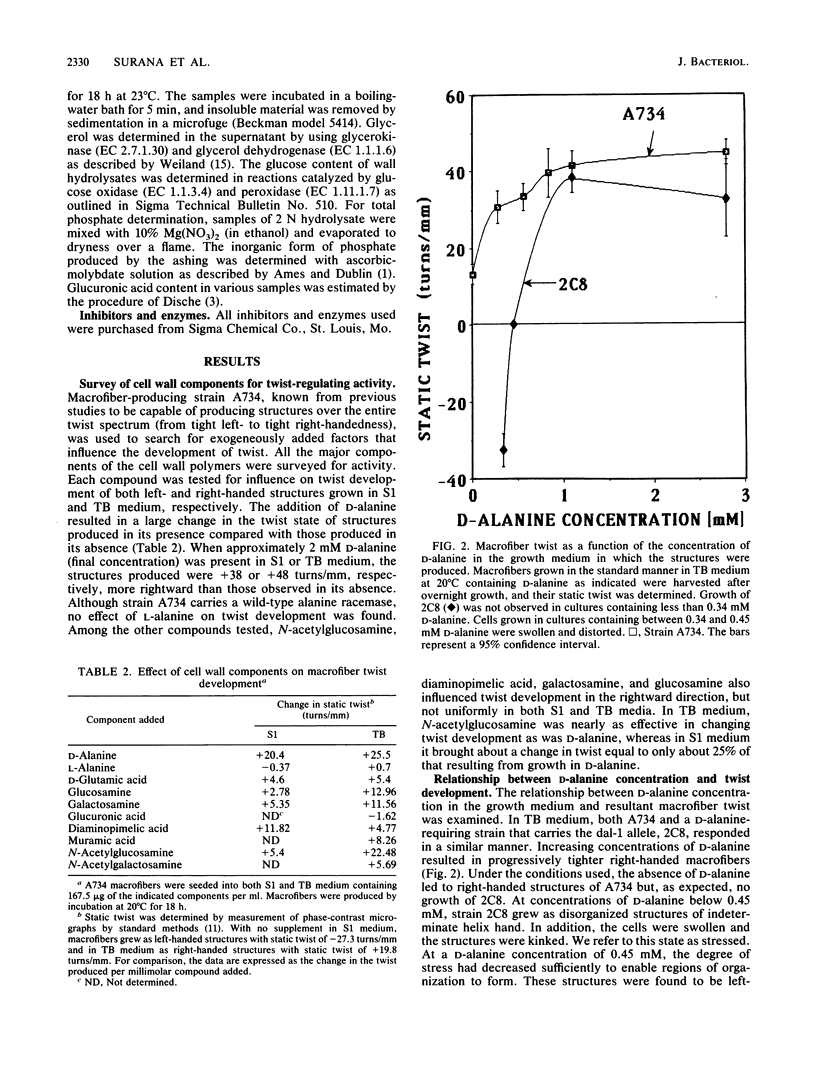
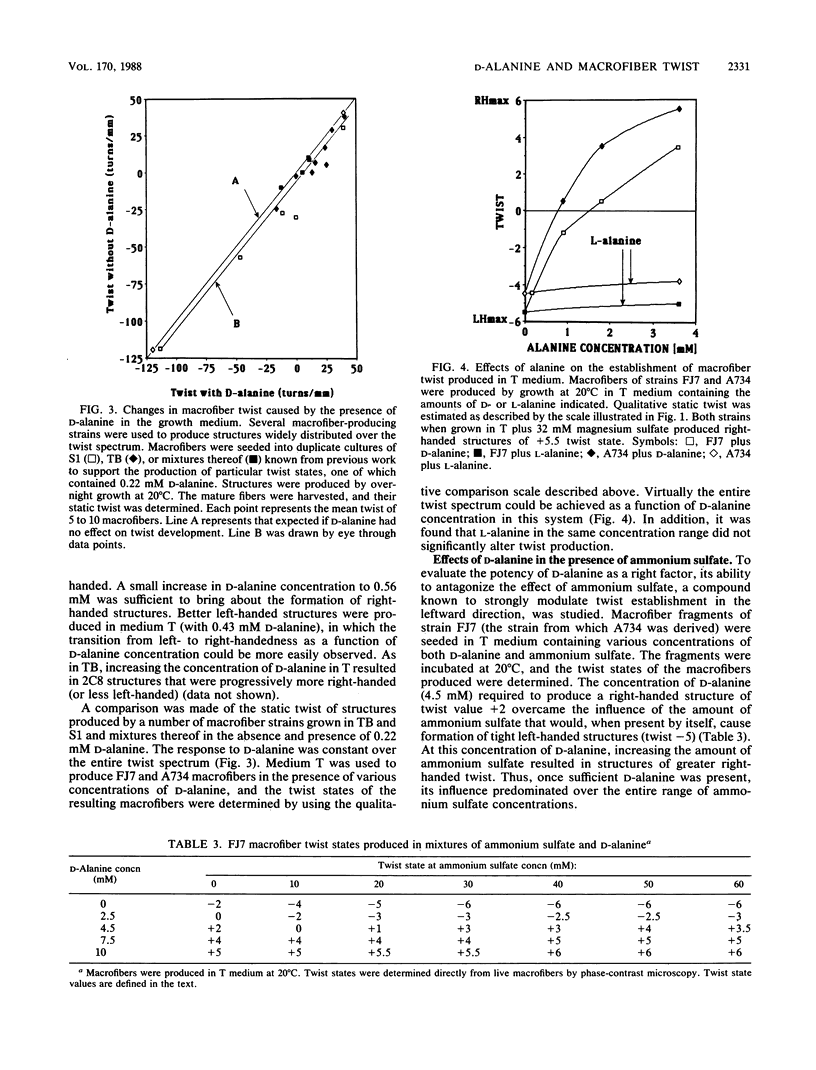
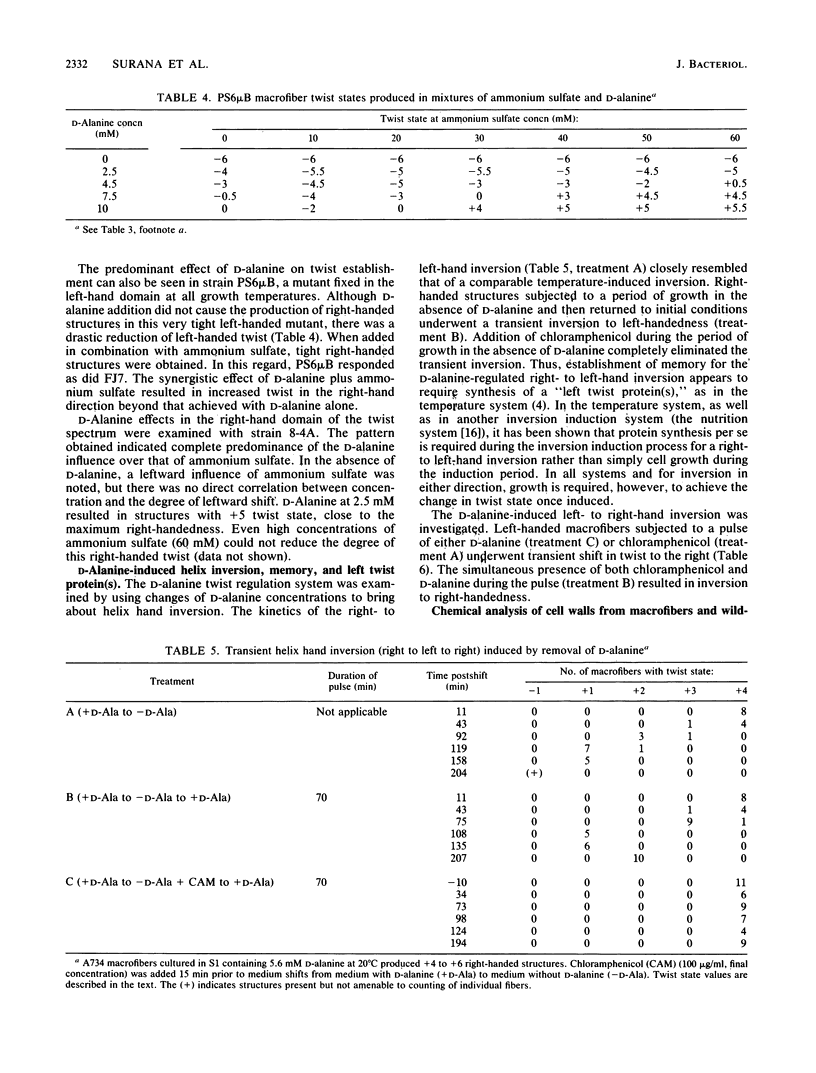
Twist states of Bacillus subtilis macrofibers were found to vary as a function of the concentration of D-alanine in the medium during growth. L-Alanine in the same concentration range had no effect. Increasing concentrations of D-alanine resulted in structures progressively more right-handed (or less left-handed). All strains examined in this study, including mutants fixed in the left-hand domain as a function of temperature, responded to D-alanine in the same way. All twist states from tight left- to tight right-handedness could be achieved solely by varying the D-alanine concentration. The D-alanine-requiring macrofiber strain 2C8, which carries a genetic defect (dal-1) in the alanine racemase, behaved in a similar fashion. The combined effects of D-alanine and ammonium sulfate (a factor known to influence macrofiber twist development in the leftward direction) were examined by using both strains able to undergo temperature-induced helix hand inversion and others incapable of doing so. In all cases, the effects of D-alanine predominated. A synergism was found in which increasing the concentration of ammonium sulfate in the presence of D-alanine enhanced the right-factor activity of the latter. A D-alanine pulse protocol provided evidence that structures undergo a transient inversion indicative of "memory." Chloramphenicol treatment inhibited the establishment of memory in the D-alanine-induced right to left inversion, supporting the existence of a "left twist protein(s)" that is required for the attainment of left-handed twist states. Chemical analysis of cell walls obtained from right- and left-handed macrofibers produced in the presence and absence of D-alanine, respectively, failed to reveal twist state-specific differences in the overall composition of either peptidoglycan or wall teichoic acids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Clark V. L., Young F. E. D-alanine incorporation into macromolecules and effects of D-alanine deprivation on active transport in Bacillus subtilis. J Bacteriol. 1978 Mar;133(3):1339–1350. doi: 10.1128/jb.133.3.1339-1350.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D., Karamata D., Mendelson N. H. Temperature-pulse-induced "memory" in Bacillus subtilis macrofibers and a role for protein(s) in the left-handed-twist state. J Bacteriol. 1985 Dec;164(3):1141–1145. doi: 10.1128/jb.164.3.1141-1145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Dynamics of Bacillus subtilis helical macrofiber morphogenesis: writhing, folding, close packing, and contraction. J Bacteriol. 1982 Jul;151(1):438–449. doi: 10.1128/jb.151.1.438-449.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Favre D. Regulation of Bacillus subtilis macrofiber twist development by ions: effects of magnesium and ammonium. J Bacteriol. 1987 Feb;169(2):519–525. doi: 10.1128/jb.169.2.519-525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Favre D., Thwaites J. J. Twisted states of Bacillus subtilis macrofibers reflect structural states of the cell wall. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3562–3566. doi: 10.1073/pnas.81.11.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Helical Bacillus subtilis macrofibers: morphogenesis of a bacterial multicellular macroorganism. Proc Natl Acad Sci U S A. 1978 May;75(5):2478–2482. doi: 10.1073/pnas.75.5.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Helical growth of Bacillus subtilis: a new model of cell growth. Proc Natl Acad Sci U S A. 1976 May;73(5):1740–1744. doi: 10.1073/pnas.73.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Karamata D. Inversion of helix orientation in Bacillus subtilis macrofibers. J Bacteriol. 1982 Jul;151(1):450–454. doi: 10.1128/jb.151.1.450-454.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Regulation of Bacillus subtilis macrofiber twist development by D-cycloserine. J Bacteriol. 1988 May;170(5):2336–2343. doi: 10.1128/jb.170.5.2336-2343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Thwaites J. J., Favre D., Surana U., Briehl M. M., Wolfe A. Factors contributing to helical shape determination and maintenance in Bacillus subtilis macrofibres. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):99–103. doi: 10.1016/s0769-2609(85)80029-6. [DOI] [PubMed] [Google Scholar]
- Wolfe A. J., Mendelson N. H. Characterization of nutrition-induced helix hand inversion of Bacillus subtilis macrofibers. J Bacteriol. 1987 Sep;169(9):4068–4075. doi: 10.1128/jb.169.9.4068-4075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



