Abstract
Objective
To establish the association between airway inflammation and severity of obstructive sleep apnoea (OSA) in children.
Methods
Consecutive children presenting with symptoms suggestive of OSA were recruited. They completed a sleep apnoea symptom questionnaire, underwent physical examination, spirometry, sputum induction and an overnight polysomnography. Adequate sputum contained <50% squamous epithelial cells, and OSA was diagnosed if the obstructive apnoea index was >1.
Results
73 children with a median (interquartile range (IQR)) age of 11.3 (10.0–13.2) years were recruited. There were 21 girls and the median body mass index of the group was 24.0 (18.0–27.0) kg/m2. The most common presenting symptoms were habitual snoring, mouth breathing and prone sleeping position. Sputum induction was successful in 43 (59%) children, of whom 14 were found to have OSA. Children with OSA had significantly greater percentage sputum neutrophil than those without OSA (18.5 (IQR 8.0–42.0) v 4 (IQR 3.0–11.3), p = 0.006). On multiple regression analysis, percentage sputum neutrophil was significantly associated with OSA (odds ratio = 1.1, p = 0.013).
Conclusion
Children with OSA had airway inflammation characterised by a marked increase in neutrophils. Further studies are needed to confirm these findings and to better define the downstream cellular interactions and molecular pathogenesis in childhood OSA.
Obstructive sleep apnoea (OSA) is characterised by repeated episodes of upper airway occlusion during sleep, which are associated with daytime behavioural changes and abnormalities in cardiovascular function.1,2,3 In children, the most common cause of OSA is adenotonsillar hypertrophy.4 The condition is being increasingly recognised, and the prevalence among the paediatric population could be as high as 10.3%.5
In adults, evidence suggests that both local airway and systemic inflammation are implicated in the pathophysiology of this seemingly all‐mechanical condition. Surgically removed tissues from patients with OSA undergoing uvulopalatopharyngoplasty and tonsillectomy showed marked subepithelial oedema6 and inflammatory cell infiltration.7 The number of leucocytes is substantially increased in the upper airway mucosa of adults with OSA, especially in the muscular layer of the soft palate and tonsillar pillars.8 Polymorphonuclear leucocytes, bradykinin and vasoactive intestinal peptide are found at increased levels in the nasal lavage fluid of patients with OSA.9 Exhaled markers of airway inflammation and oxidative stress such as interleukin 6 and 8‐isopentane are also found at raised levels in the breath condensate of adults with OSA.10,11 Serum tumour necrosis factor α, interleukin 6 and C reactive protein have all been found at higher levels in adults with OSA than in controls.12,13 Treatment of OSA improves the daytime symptoms of the patients and also decreases the risk of cardiovascular complications. These treatment effects are postulated to be related to decreases in systemic inflammation. In support of this hypothesis, serum C reactive protein has been found to reduce considerably after 1 month of nasal continuous positive airway pressure treatment.14
Accumulating evidence suggests that local and systemic inflammatory responses also exist in children with OSA.15,16 C reactive protein levels are raised in children with OSA, which may suggest ongoing systemic inflammation contributing to the proliferation of the upper airway lymphoid tissues.15 Cysteinyl leucotrienes are major mediators of inflammation, potent neutrophil chemoattractants and activators.17 Up regulation of cysteinyl leucotriene receptor has been found in the tonsils of children undergoing tonsillectomy for OSA.18 Montelukast, a cysteinyl leckotriene receptor antagonist, induced reductions in adenoidal size and respiratory‐related sleep disturbances.19 However, data are not available on airway inflammation and its correlation with OSA severity in children. If such a relationship does exist, it would be interesting to examine the role of anti‐inflammatory treatment for OSA in childhood.
Sputum induction has gained popularity over the past few years as a non‐invasive method in assessing airway inflammation. The technique is well established and safe to use in children.20 In this study, we investigated airway inflammation in children with OSA by using the analysis of differential cell counts in induced sputum.
Methods
Subjects
Children with symptoms suggestive of OSA and aged 6–18 years were consecutively recruited from the Paediatric Respiratory, Sleep Disorder and Obesity Clinic, Prince of Wales Hospital, The Chinese University of Hong Kong SAR, during the period between January 2004 and June 2005. Patients were excluded from the study if they had an intercurrent respiratory tract infection, a neuromuscular disorder such as Duchenne's muscular dystrophy, craniofacial anomalies or a syndromic disorder—such as Down's syndrome, or if they had previously undergone upper airway surgery. The study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong, Shatin, Hong Kong SAR, and informed written consent was obtained at the beginning of the assessment from the children and their parents. Each child underwent physical examination on the day of admission and overnight polysomnography (PSG). Lung function tests and sputum induction were carried out in the morning after the overnight PSG.
Anthropometry and physical assessment
The weight and standing height of the children were measured with a calibrated weighing scale and stadiometer, respectively, by standard anthropometric methods.21 Body mass index was calculated as weight/height2 (kg/m2). The sizes of the tonsils were graded according to a standardised scale from 0 (absent) to 4 (kissing tonsils).22,23
Lung function
Lung function tests were performed using a standard technique to measure the forced expiratory volume in the first second and forced vital capacity (Spirolab II, MIR, Rome, Italy).24 The best of three efforts was compared with local reference values matched for age and sex.25
Sputum induction and processing
Sputum induction involved the incremental inhalation of a 4.5% hypertonic saline solution via an ultrasonic nebuliser, as described previously.20 Sputum induction was performed only if the child's forced expiratory volume in the first second was >65% predicted. Sputum differential cell counts were evaluated by a laboratory investigator blinded to the clinical data of the children. Specimens containing squamous epithelial cells of <50% of the total inflammatory cell number were considered to be successful inductions. At least 400 inflammatory cells were counted for each specimen, and the percentage of eosinophils, neutrophils, macrophages and lymphocytes was recorded.
Sleep study
An overnight PSG was performed on each child using the Siesta ProFusion II PSG monitor (Compumedics Telemed, Abbotsford, Victoria, Australia), as described previously.26 Obstructive apnoea was defined as absence of air flow with persistent respiratory effort lasting longer than two baseline breaths, irrespective of changes in oxygen saturation. Obstructive Apnoea Index (OAI) was defined as the number of obstructive apnoea episodes per hour of sleep. Central Apnoea was defined as absence of respiratory effort associated with absence of air flow. Apnoea lasting longer than 20 s with or without oxygen desaturation or arousals, and those of any duration but associated with oxygen desaturation of at least 4% and/or arousals are quantified. Hypopnoea was defined as a reduction of ⩾50% in the amplitude of the air flow signal. It was quantified only if longer than two baseline breaths and associated with oxygen desaturation of at least 4% and/or arousals. Apnoea Hypopnoea Index was defined as the total number of apnoeic and hypopnoeic episodes per hour of sleep. Oxygen saturation nadir and the percentage of total sleep time where oxygen saturation was <90% were noted. Arousal was defined as an abrupt shift in electroencephalographic frequency during sleep, which may include theta, alpha and/or frequencies >16 Hz but not spindles, with 3–15 s in duration. In rapid eye movement sleep, arousals were scored only when accompanied by concurrent increases in the submental electromyographic amplitude. We defined OSA as OAI >1.0. Children who were taking an antihistamine on an as‐required basis were asked to withhold their drug for 48 h before undergoing PSG.
Statistical analysis
The results are presented as medians with interquartile ranges (IQR). Potential factors including demographic data, PSG parameters, lung function measures and sputum differential cell counts were evaluated for their association with OSA using the Mann–Whitney U test and χ2 test. Risk factors with a p <0.25 were then analysed by univariate and multivariate logistic regression analyses, using a forward stepwise selection strategy. The most significant factor (ie, the one that would result in the largest likelihood ratio statistic) was added to the model at each step, and the process was continued until no further significant contributing factor could be added. Whenever two or more potential factors were highly correlated or had p values that were similar, the factor that was more clinically or biologically important was selected for entry. The relationship between airway inflammation and severity of OSA was presented after logarithmic transformation. Analyses were performed using SPSS V.13.0 for Windows. The level of significance was set at 5% in all comparisons.
Results
Seventy three consecutive children were invited and agreed to participate in this study. There were 52 boys and the median (IQR) age of the children was 11.3 (10.0–13.2) years. Forty nine children had allergic rhinitis for which they used an antihistamine on an as‐required basis, and six were regularly using a corticosteroid nasal spray. One child had asthma and 17 had a combination of allergic rhinitis and asthma, but none of the children with asthma was taking regular inhaled corticosteroids and their asthma had been well controlled before and during this study. All children had normal physical examination and pulmonary function tests; forced expiratory volume in the first second and forced vital capacity were 94% predicted (83.3–100.8) and 93% predicted (84.3–100.8), respectively. The three most common OSA‐related presenting complaints were habitual snoring (snoring for >3 nights per week), mouth breathing and prone sleeping position. All children completed the sputum induction protocol and an adequate sputum sample was obtained in 43 (59%) children. None of the children complained of any adverse effects from the procedure. Table 1 shows the characteristics of the children with and without successful sputum induction. Those who were able to produce an adequate sputum sample were considerably older and taller.
Table 1 Characteristics of children with and without successful sputum induction.
| With sputum (n = 43)* | No sputum (n = 30)* | p Value | |
|---|---|---|---|
| Age (years) | 11.8 (1.08–13.7) | 10.6 (8.8–11.6) | 0.005 |
| Male/female (n) | 30/13 | 22/8 | 0.742 |
| Weight (kg) | 53.4 (40.5–66.0) | 42.0 (29.6–59.9) | 0.071 |
| Height (m) | 1.5 (1.4–1.6) | 1.4 (1.3–1.5) | 0.008 |
| BMI (kg/m2) | 24.3 (20.7–27.4) | 22.8 (15.2–26.8) | 0.239 |
| Tonsil size, left | 1.0 (1.0–3.0) | 1.0 (1.0–2.0) | 0.617 |
| Tonsils size, right | 1.0 (0–3.0) | 1.0 (1.0–2.0) | 0.981 |
| FEV1 (% predicted) | 93.5 (84.0–100.0) | 97.0 (82.5–101.0) | 0.846 |
| FVC (% predicted) | 92.5 (85.0–100.0) | 95.0 (81.0–102.8) | 0.871 |
| FEV1/FVC | 90.0 (85.0–94.0) | 91.0 (90.0–94.0) | 0.247 |
| OAI | 0.2 (0.0–1.3) | 0.3 (0.0–1.4) | 0.592 |
| AHI | 2.0 (0.7–5.5) | 1.4 (0.2–5.4) | 0.340 |
| %TST with SaO2 <90% | 1.0 (0–0.03) | 2.5 (0.2–6.7) | 0.561 |
| SaO2 nadir (%) | 87.5 (83.0–91.0) | 89.0 (77.5–90.3) | 0.991 |
| Arousal index | 6.7 (4.9–11.2) | 6.0 (3.9–10.4) | 0.349 |
| Asthma, yes/no (n) | 12/31 | 6/24 | 0.444 |
| Allergic rhinitis, yes/no (n) | 30/13 | 19/11 | 0.567 |
AHI, Apnoea Hypopnoea Index; BMI, body mass index; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; OAI, obstructive apnoea index; SaO2, oxygen saturation; %TST with SaO2 <90%: percentage of total sleep time with oxygen saturation <90%.
*Values are median (IQR).
In all, 24 children satisfied the diagnostic criteria for OSA, of whom 14 had successful sputum induction. Among children with successful sputum induction, the characteristics of those with and without OSA were compared (table 2). We found no significant differences in age, sex distribution, anthropometric measures, pulmonary function and tonsil sizes between the two groups. Children with OSA had significantly higher percentage sputum neutrophils (18.5 (8.0–42.0) v 4.0 (3.0–11.3), p = 0.006) and lower percentage sputum macrophages (76.0 (56.0–87.0) v 84.0 (77.8–89.0), p = 0.015) and eosinophils (0.4 (0–3.0) v 2.0 (0.8–4.2), p = 0.023) than their counterparts without OSA. We found no significant differences in the number of children with and without asthma (p = 0.279) and allergic rhinitis (p = 1.00) between those with and without OSA.
Table 2 Characteristics of children with and without obstructive sleep apnoea (OSA).
| Without OSA (n = 29)* | With OSA (n = 14)* | p Value | |
|---|---|---|---|
| Age (years) | 11.6 (10.8–13.8) | 11.8 (11.0–13.6) | 0.678 |
| Male/female (n) | 22/7 | 8/6 | 1.000 |
| Weight (kg) | 52.3 (39.7–64.4) | 53.4 (48.3–66.0) | 0.577 |
| Height (m) | 1.5 (1.4–1.5) | 1.5 (1.5–1.6) | 0.508 |
| BMI (kg/m2) | 24.3 (19.8–27.3) | 24.3 (20.9–30.0) | 0.836 |
| Tonsil size, left | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) | 0.126 |
| Tonsils size, right | 1.0 (0.0–2.0) | 1.5 (1.0–4.0) | 0.089 |
| FEV1 (% predicted) | 94.0 (87.0–102.5) | 87.0 (82.8–96.0) | 0.187 |
| FVC (% predicted) | 95.0 (85.0–100.3) | 89.0 (84.8–99.0) | 0.531 |
| FEV1/FVC | 90.0 (88.0–94.3) | 86.0 (84.0–93.5) | 0.130 |
| Sputum eosinophils (%) | 2.0 (0.8–4.2) | 0.4 (0.0–3.0) | 0.023 |
| Sputum neutrophils (%) | 4.0 (3.0–11.3) | 18.5 (8.0–42.0) | 0.006 |
| Sputum macrophages (%) | 84.0 (77.8–89.0) | 76.0 (56.0–87.0) | 0.015 |
| Sputum lymphocytes (%) | 6.8 (2.0–8.0) | 2.5 (1.0–9.7) | 0.413 |
| %TST with SaO2 <90% | 0.1 (0.0–0.2) | 1.6 (0.2–3.7) | 0.008 |
| SaO2 nadir (%) | 90.5 (84.3–93.0) | 82.0 (72.0–87.0) | <0.001 |
| Arousal index per h | 5.7 (4.4–8.3) | 9.1 (6.8–15.3) | 0.010 |
| Asthma, yes/no (n) | 10/19 | 2/12 | 0.279 |
| Allergic rhinitis, yes/no (n) | 21/8 | 9/5 | 1.000 |
AHI, Apnoea Hypopnoea Index; BMI, body mass index; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; OAI, obstructive apnoea index; SaO2, oxygen saturation; %TST with SaO2 <90%: percentage of total sleep time with oxygen saturation <90%.
*Values are median (IQR) unless otherwise stated.
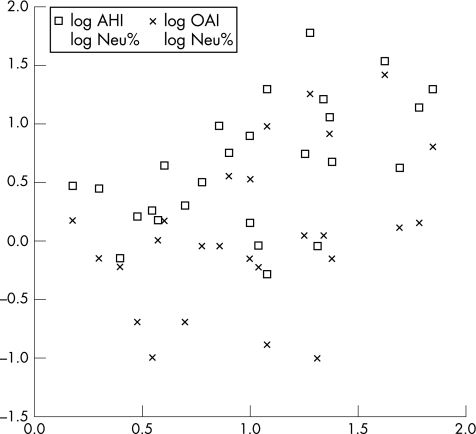
Table 3 shows the univariate analysis demonstrating significantly different parameters between OSA and non‐OSA groups. On multiple regression analysis, only percentage sputum neutrophil (odds ratio 1.10 (95% confidence interval (CI) 1.02 to 1.18), p = 0.013 and oxygen saturation nadir (0.85 (0.75 to 0.96), p = 0.007) remained as significant predictors for OSA. Figure 1 shows the scatter plot of relationships between obstructive apnoea index/Apnoea Hypopnoea Index and sputum neutrophils after logarithmic transformation.
Table 3 Univariate logistic regression.
| Without OSA (n = 29)* | With OSA (n = 14)* | OR (95% CI) | p Value | |
|---|---|---|---|---|
| Tonsil size, left | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) | 1.51 (0.91 to 2.48) | 0.110 |
| Tonsils size, right | 1.0 (0.0–2.0) | 1.5 (1.0–4.0) | 1.62 (0.98 to 2.98) | 0.098 |
| FEV1 (% predicted) | 94.0(87.0–102.5) | 87.0 (82.8–96.0) | 0.96 (0.89 to 1.03) | 0.233 |
| Sputum eosinophils (%) | 2.0 (0.8–4.2) | 0.4 (0.0–3.0) | 0.67 (0.44 to 1.03) | 0.067 |
| Sputum neutrophils (%) | 4.0 (3.0–11.3) | 18.5 (8.0–42.0) | 1.08 (1.02 to 1.16) | 0.014 |
| Sputum macrophages (%) | 84.0 (77.8–89.0) | 76.0 (56.0–87.0) | 0.93 (0.88 to 0.99) | 0.017 |
| %TST with SaO2 <90% | 0.1 (0.0–0.2) | 1.6 (0.2–3.7) | 1.79 (1.01 to 3.18) | 0.046 |
| SaO2 nadir (%) | 90.5 (84.3–93.0) | 82.0 (72.0–87.0) | 0.87 (0.78 to 0.96) | 0.004 |
| Arousal index | 5.7 (4.4–8.3) | 9.1 (6.8–15.3) | 1.25 (1.04 to 1.51) | 0.020 |
| Asthma, yes/no (n) | 10/19 | 2/12 | 3.18 (0.59 to 16.96) | 0.180 |
FEV1, forced expiratory volume in the first second; OSA, obstructive sleep apnoea; SaO2, oxygen saturation; %TST with SaO2 <90%: percentage of total sleep time with oxygen saturation <90%.
*Values are median (IQR) unless otherwise mentioned.
Figure 1 Relationship between obstructive apnoea index (OAI)/Apnoea Hypopnoea Index (AHI) and sputum neutrophil (Neu).
Discussion
We have shown for the first time that in children with OSA, percentage sputum neutrophils were markedly increased and the degree of neutrophilic airway inflammation correlated significantly and positively with the severity of OSA.
Using specific histological parameters and surrogate biomarkers in the nasal lavage fluid and exhaled breath, previous studies have collectively shown that nasal and oropharyngeal mucosal inflammation is present in patients with OSA.6,7,8,9,10,11 Recurrent vibration in the upper airway of children with OSA has been suggested to promote such inflammatory changes in the tonsillar tissue and upper airway mucosa.27 The stimulation of any part of the respiratory tract can have ripple effects along the entire airway, and this reinforces the “united airways” concept.28 A case was recently reported involving a healthy patient with cystic fibrosis who developed recurrent cough with worsening lower airway obstruction that was reversed after treatment for OSA.29 This observation could be explained by the “united airways” hypothesis, whereby OSA causes airway inflammation that adversely affects lung function. Neutrophilic airway inflammation was shown in adult patients with OSA.30 In a study on 16 patients with OSA, Salerno et al30 found a significantly higher percentage of neutrophils in the induced sputum of patients than in healthy volunteers (mean (standard deviation) 66.7 (18.9) v 25.8 (15.6), p<0.001). The percentage sputum neutrophil count obtained in our current study is not as high as that shown in adult patients, and differences in the chronicity and severity of disease among adults could explain this discrepancy. Adult patients tend to have had prolonged disease before diagnosis and they typically have more severe OSA than their paediatric counterparts. In another study on 19 male patients with OSA, circulating neutrophil levels were higher in affected patients than in controls.31 In addition, receptors of cysteinyl leucotrienes, which are potent neutrophil chemoattractants and activators, were found at increased levels in the tonsils of children undergoing tonsillectomy for OSA.17,18
Limited data are available evaluating the effects of anti‐inflammatory treatment on upper airway inflammation in OSA. Topical nasal corticosteroids produced only modest therapeutic effect in children with OSA, without any marked change in the size of the adenoid or tonsillar tissues.32 This may be at least partly explained by the fact that corticosteroids are better at controlling eosinophilic than neutrophilic inflammation. It has recently been reported that montelukast, a cysteinyl leucotriene receptor antagonist, induced considerable reductions in adenoidal size and respiratory‐related sleep disturbances in children with OSA.19 Taken together, there is accumulating evidence to support an inflammatory neutrophilic reaction in the airways of patients with OSA.
Current research has highlighted the potential use of sputum induction as a non‐invasive method of assessing airway inflammation. The availability of both sputum cellular and fluid components for identifying types of inflammatory cells and pattern of cytokines and chemokines has allowed a better delineation of the underlying inflammatory process.33,34 The success rate of sputum induction in children varies from 68% to 100%, and the procedure is generally well tolerated.20 If our findings can be confirmed in subsequent studies involving a larger sample size, then sputum examination may be used as an objective assessment of the severity of OSA or as a guide to evaluate success of both medical and surgical interventions.
Our study has a few limitations. (1) Our study was a cross‐sectional assessment at a single time point. Longitudinal data including interventions would have provided more interesting data for the association and complex interaction between OSA and airway inflammation. (2) We have selected a biased sample as we recruited our cohort from attendants at our Paediatric Respiratory, Sleep Disorder and Obesity Clinic. Boys are more likely to be obese and this explains the male preponderance seen in this study. We had excluded those children aged <6 years as in our experience it is more difficult to induce sputum in children under this age. Further studies on more children with typical OSA will be needed to confirm the association between airway inflammation and severity of OSA seen in our study.
Our study has provided a new insight into the pathophysiology of OSA in children, highlighting the presence of neutrophilic inflammation in the lower airways. Further studies are needed to confirm our findings and to better define the downstream cellular interactions and molecular pathogenesis in childhood OSA.
Abbreviations
OSA - obstructive sleep apnoea
PSG - polysomnography
Footnotes
Funding: This study received a direct grant for research from the Chinese University of Hong Kong (reference number 2005.2.028).
Competing interests: None.
References
- 1.Brouillette R T, Fernbach S K, Hunt C E. Obstructive sleep apnea in infants and children. J Pediatr 198210031–40. [DOI] [PubMed] [Google Scholar]
- 2.Amin R S, Carroll J L, Jeffries J L.et al Twenty‐four‐hour ambulatory blood pressure in children with sleep‐disordered breathing. Am J Respir Crit Care Med 2004169950–956. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D. Sleep‐disordered breathing and school performance in children. Pediatrics 1998102616–620. [DOI] [PubMed] [Google Scholar]
- 4.Arens R, McDonough J M, Corbin A M.et al Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 200316765–70. [DOI] [PubMed] [Google Scholar]
- 5.Redline S, Tishler P V, Schluchter M.et al Risk factors for sleep‐disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med 19991591527–1532. [DOI] [PubMed] [Google Scholar]
- 6.Saul S, Kimmelman C P, Brooks J S.et al Histopathology of sleep apnea. Trans Am Laryngol Assoc 1988109222–225. [Google Scholar]
- 7.Sekosan M, Zakkar M, Wenig B.et al Inflammation in the uvula mucosa of patients with obstructive sleep apnea. Laryngoscope 19961061018–1020. [DOI] [PubMed] [Google Scholar]
- 8.Boyd J H, Petrof B J, Hamid Q.et al Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med 2004170541–546. [DOI] [PubMed] [Google Scholar]
- 9.Rubinstein I. Nasal inflammation in patients with obstructive sleep apnea. Laryngoscope 1995105175–177. [DOI] [PubMed] [Google Scholar]
- 10.Olopade C O, Christon J A, Zakkar M.et al Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest 19971111500–1504. [DOI] [PubMed] [Google Scholar]
- 11.Carpagnano G E, Kharitonov S A, Resta O.et al Increased 8‐isoprostane and interleukin‐6 in breath condensate of obstructive sleep apnea patients. Chest 20021221162–1167. [DOI] [PubMed] [Google Scholar]
- 12.Entzian P, Linnemann K, Schlaak M.et al Obstructive sleep apnea and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med 19961531080–1086. [DOI] [PubMed] [Google Scholar]
- 13.Shamsuzzaman A S, Winnicki M, Lanfranchi P.et al Elevated C‐reactive protein in patients with obstructive sleep apnea. Circulation 20021052462–2464. [DOI] [PubMed] [Google Scholar]
- 14.Yokoe T, Minoguchi K, Matsuo H.et al Elevated levels of C‐reactive protein and interleukin‐6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 20031071129–1134. [DOI] [PubMed] [Google Scholar]
- 15.Tauman R, Ivanenko A, O'Brien L M.et al Plasma C‐reactive protein levels among children with sleep‐disordered breathing. Pediatrics 2004113564–569. [DOI] [PubMed] [Google Scholar]
- 16.Larkin E K, Rosen C L, Kirchner H L.et al Variation of C‐reactive protein levels in adolescents: association with sleep‐disordered breathing and sleep duration. Circulation 20051111978–1984. [DOI] [PubMed] [Google Scholar]
- 17.Nohgawa M, Sasada M, Maeda A.et al Leukotriene B4 activated human endothelial cells promote transendothelail neutrophil migration. J Leukoc Biol 199762203–209. [DOI] [PubMed] [Google Scholar]
- 18.Goldbart A D, Goldman G L, Li R C.et al Differential expression of cysteinyl leukotriene receptors 1 and 2 in tonsils of children with obstructive sleep apnea and recurrent infection. Chest 200412613–18. [DOI] [PubMed] [Google Scholar]
- 19.Goldbart A D, Goldman G L, Veling M C.et al Leukotriene modifier therapy for mild sleep‐disordered breathing in children. Am J Respir Crit Care Med 2005172364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li A M, Tsang T W T, Chan D F Y.et al Sputum induction in children with asthma: a tertiary center experience. Pediatr Pulmonol 200641720–725. [DOI] [PubMed] [Google Scholar]
- 21.Tanner J M. Physical growth and development. In: Forfar JO, Arneil GC, eds. Textbook of paediatrics. Edinburgh: Churchill Livingstone, 1984304–305.
- 22.Brooks L J, Stephens B M, Bacevice A M. Adenoid size is related to severity but not the number of episodes of obstructive apnea in children. J Pediatr 1998132682–686. [DOI] [PubMed] [Google Scholar]
- 23.Leach J, Olson J, Hermann J.et al Polysomnographic and clinical findings in children with obstructive sleep apnea. Arch Otolaryngol 1992118741–744. [DOI] [PubMed] [Google Scholar]
- 24.Medical Section of the American Lung Association Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 19951521107–1136. [DOI] [PubMed] [Google Scholar]
- 25.Ip M S, Karlberg E M, Karlberg J P.et al Lung function reference values in Chinese children and adolescents in Hong Kong. Am J Respir Crit Care Med 2000162424–429. [DOI] [PubMed] [Google Scholar]
- 26.Li A M, Wing Y K, Cheung A.et al Is a 2‐night polysomnographic study necessary in childhood sleep‐related disordered breathing? Chest 20041261467–1472. [DOI] [PubMed] [Google Scholar]
- 27.Cohn M, Hesla P E, Kiel M.et al Vibration frequency of snoring in obstructive sleep apnea syndrome. Chest 198689529S [Google Scholar]
- 28.McCusker C T. Use of mouse models of allergic rhinitis to study the upper and lower airway link. Curr Opin Allergy Clin Immunol 2004411–16. [DOI] [PubMed] [Google Scholar]
- 29.Hayes D. Obstructive sleep apnea syndrome: a potential cause of lower airway obstruction in cystic fibrosis. Sleep Med 2006773–75. [DOI] [PubMed] [Google Scholar]
- 30.Salerno F G, Carpagnano E, Guido P.et al Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med 20049825–28. [DOI] [PubMed] [Google Scholar]
- 31.Ryan S, Taylor C T, McNicholas W T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 20051122660–2667. [DOI] [PubMed] [Google Scholar]
- 32.Brouillette R T, Manoukian J J, Ducharme F M.et al Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr 2001138838–844. [DOI] [PubMed] [Google Scholar]
- 33.Pavord I D, Pizzichini M M, Pizzichini E.et al The use of induced sputum to investigate airway inflammation. Thorax 199752498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson P G, Henry R L, Thomas P. Noninvasive assessment of airway inflammation in children: induced sputum, exhaled nitric oxide, and breath condensate. Eur Respir J 2000161008–1015. [PubMed] [Google Scholar]