Abstract
Background
Normal adult articular cartilage is thought to be avascular and aneural.
Objective
To describe neurovascular structures at the osteochondral junction and in osteophytes in tibiofemoral osteoarthritis (OA) displaying a range of severity of cartilage changes.
Methods
Articular surfaces were obtained from 40 patients at total knee joint replacement surgery for tibiofemoral OA (TKR) and seven patients post mortem (PM). Antibodies directed against CD34 (vascular endothelium), protein gene product 9.5 (pan‐neuronal marker), substance P and calcitonin gene‐related peptide (sensory nerves) and C‐flanking peptide of neuropeptide Y (sympathetic nerves) were used to localise blood vessels and nerves by immunohistochemistry. Severity of OA cartilage changes was graded histologically.
Results
TKR and PM samples displayed a range of OA cartilage changes including tidemark breaching by vascular channels. Sympathetic and sensory nerves were both present within vascular channels in the articular cartilage, in both mild and severe OA. Perivascular and free nerve fibres, and nerve trunks were observed within the subchondral bone marrow and within the marrow cavities of osteophytes. Sensory and sympathetic nerves displayed similar distributions in each region studied.
Conclusion
Vascularisation and the associated innervation of articular cartilage may contribute to tibiofemoral pain in OA across a wide range of structural disease severity.
Tibiofemoral osteoarthritis (OA) is an increasing source of pain, disability and distress in an ageing population.1 Structural changes in the osteoarthritic articular cartilage include proteoglycan depletion, fissuring and tidemark duplication. Blood vessels have been reported to be absent from normal adult articular cartilage. However, channels containing cellular elements, including blood vessels, invade from the subchondral bone into the calcified cartilage and breach the tidemark in OA.2,3 Vascular invasion is also thought to be important in osteophyte formation.4
Pain in OA may originate from several sources, including periosteum,5 subchondral bone,6 synovium,7 ligaments and muscle.1 However, normal articular cartilage is insensate.8 Many patients with OA describe a sustained burning pain, which is characteristic of pain mediated by fine unmyelinated afferent nerve fibres.9 Unmyelinated nerves are most commonly perivascular and can be classified as either sensory or sympathetic by their neuropeptide content. Sensory nerves may contain the neuropeptides, substance P and calcitonin‐gene‐related peptide (CGRP), whereas sympathetic nerves contain the C‐flanking peptide of neuropeptide Y (CPON). Innervation has been described in detail in synovium from knees of patients with OA, and also in periosteum, menisci, cruciate and collateral ligaments, and in the joint capsule.10,11,12,13,14,15,16 The hard tissues of the human knee have proved more difficult to investigate because of technical limitations of immunohistochemistry on fixed, decalcified pathological specimens.
Substance P has been localised to nerve fibres within osteophytes and in vascular channels in the articular cartilage of proximal phalanges from horses with metacarpophalangeal OA.17 Sensory and sympathetic nerves have been localised to the subchondral bone marrow in patients and most other species, either with or without OA,16,18,19 supporting the hypothesis that subchondral bone may contribute to OA pain. Substance P‐immunoreactive nerves have also been demonstrated in vascular channels in patellar articular cartilage from patients with anterior knee pain and in lumbar facet joints from patients with low back pain.16,18 The potential for osteophytes and articular cartilage to be a source of pain in tibiofemoral OA in man remains incompletely understood.
Most unmyelinated nerves in articular tissues are associated with blood vessels. Nerves grow along blood vessels after angiogenesis in subcutaneous tissues and during callus formation.20,21 We hypothesised that, in tibiofemoral OA, innervation of the articular cartilage and osteophytes is associated with their invasion by blood vessels and nerves. We have localised blood vessels and nerves to the osteochondral junction and osteophytes at the human tibiofemoral joint, and evaluated their relationships with the severity of OA cartilage change. To investigate a broader range of OA severity than seen in patients presenting for total knee replacement surgery, we included samples from recently deceased patients with a range of severity of histological OA cartilage changes.
Materials and methods
Patients and sample preparation
Approval was obtained from the North Nottinghamshire Health Authority Local Research Ethics Committee (projects NNHA/420, NNHA/544 and NNHA/673). After informed consent, articular surfaces were collected from 40 patients fulfilling American College of Rheumatology revised criteria for tibiofemoral OA at the time of total knee joint replacement (TKR) surgery.22 Both tibial plateaux and femoral condyles were processed from eight cases, and medial tibial plateaux alone were processed from the remaining 32 cases. Samples from three medial tibial plateaux were rejected because no articular cartilage was present in the mid‐coronal section. Nine medial tibial plateaux were also collected post mortem (PM) from seven recently deceased patients (six male). TKR samples used in the study were from 37 patients (22 male) with a median age of 69 (range 42–89) years. The deceased patients (PM cases) had a median age of 77 (range 76–88.5) years and did not have rheumatoid arthritis or other clinical inflammatory joint disease as determined by case notes review and interview with the bereaved relatives. It was expected that OA would be prevalent in PM cases, but with less severity than in patients undergoing joint arthroplasty for OA (table 1).
Table 1 Clinical details and observations of nerves and vessels breaching the tidemark in post‐mortem cases.
| Case | Age | Sex | Cause of death | History of knee pain (+/–) | Heberdens nodes (+/–) | NSAID usage (+/–) | Mankin score | Tidemark breach (+/–) | PGP nerve (+/−) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | Myocardial infarction and aneuryism | – | + | – | 5 | – | – |
| 2 | 89 | M | Pulmonary oedema | – | – | – | 7 | – | – |
| 3 | 71 | M | Cerebrovascular accident | + | – | + | 2 | – | – |
| 4 (both knees) | 77 | M | Acute coronary syndrome | – | – | – | 5/9 | –/– | –/– |
| 5 | 90 | M | Pneumonia | – | – | + | 5 | – | – |
| 6 (both knees) | 88 | F | Cerebrovascular accident | – | – | – | 4/7 | +/– | +/– |
| 7 | 77 | M | Rectal tumour | – | – | – | 10 | – | – |
NSAID, non‐steroidal anti‐inflammatory drug; PGP, protein gene product.
Coronal slices were taken of the mid‐articular surface from the tibial plateaux and femoral condyles. All samples were fixed in Zamboni's solution (2% (w/v) paraformaldehyde, 15% (v/v) picric acid in phosphate buffer, pH 7.3)23 overnight at 4°C and then transferred to 15% (w/v) sucrose in phosphate‐buffered saline (PBS/sucrose) solution at 4°C for 5 days. Decalcification of the fixed blocks took place over a period of 3 weeks in 10% (w/v) EDTA (sodium salt)/7.5% (w/v) polyvinylpyrrolidone in 0.01 M Tris base, pH 6.95, at 4°C. The samples were considered decalcified when no further mineral was detectable by radiography. Samples were transferred to PBS/sucrose, then a 1:1 mixture of PBS/sucrose and OCT, and then 100% OCT for a further 7 days. Each sample was cut into three equal‐sized pieces (“joint margin”, “central” and “adjacent to cruciate ligament attachment”). Each piece was mounted in OCT and then snap‐frozen in melting isopentane and stored at −80°C. Central pieces were used for analysis of the osteochondral junction, and joint margin pieces for osteophyte analyses.
A total of five TKR cases and five PM cases were used for comparative, compartmental analysis of nerve and vessel distribution (medial and lateral tibial plateaux, medial and lateral femoral condyles). The 15 joint margin samples displaying the largest osteophytes were selected for immunohistochemical analysis of nerves in osteophytes. Synovium from patients undergoing TKR for OA, containing intimal lining and deep sublining tissue, was fixed in Zamboni's solution as above, cryopreserved in PBS/sucrose, mounted in OCT, and frozen in melting isopentane, as above but without decalcification; this provided positive control tissue for immunohistochemistry.
Safranin O staining and scoring for severity of OA cartilage changes
Tissue sections (5 μm) were stained with safranin O. After nuclear staining with Weigert's haematoxylin (2 min) and dedifferentiation in acid alcohol (15 s), sections were immersed in 0.02% fast green FCF, then 0.1% safranin O for 1 min, and mounted in dibutyl phthalate polystyrene xylene. Severity of OA changes in the articular cartilage was evaluated using the method of Mankin, in which the overall structure, cell morphology, safranin O staining and tidemark integrity of the sample were each assessed by an observer who was blinded to clinical details and innervation status.24 Severity scores can range between 0 (no observed changes) and 14 (most severe).
Immunohistochemical analysis
Tissue sections (15 μm) were cut in a cryostat maintained at −30°C and thaw‐mounted on to Superfrost slides. Vascular endothelium was localised by indirect immunohistochemistry using a mouse monoclonal antibody to CD34 (clone QBEnd 10), a horse anti‐mouse biotinylated secondary antibody, and alkaline phosphatase‐labelled ABC, visualised using FastRed. Nerves were immunolocalised using rabbit polyclonal antibodies to the pan‐neuronal marker, protein gene product (PGP) 9.5, the sensory neuropeptides, CGRP and substance P, and the sympathetic nerve marker, CPON. A goat anti‐rabbit biotinylated secondary antibody was used with peroxidase‐labelled ABC, visualised by the nickel‐enhanced diaminobenzidene method.25 Sections were dehydrated and mounted in dibutyl phthalate polystyrene xylene.
Samples were characterised as containing one or more nerves by screening five serial sections using immunohistochemistry for PGP 9.5. PGP 9.5‐immunoreactive samples were further analysed by immunohistochemistry for sensory and sympathetic nerve markers.
Microscopic analysis
Transmitted light microscopy of non‐counterstained preparations was used to identify immunoreactive nerves. Autofluorescence of these preparations under UV light (420 nm) allowed discrimination of calcified and non‐calcified cartilage, the tidemark and subchondral bone. FastRed reaction product was detected by transmitted light microscopy and also using its fluorescent properties under UV illumination (615 nm).
Increasing objective magnifications (×20 and ×40) were used to identify nerves. The investigator sequentially observed each consecutive field along the osteochondral junction of the entire tissue section, fine‐focusing through the 15 μm depth of the tissue. Boundaries were determined which enabled qualitative and quantitative determination of the location of nerve fibres within calcified cartilage, non‐calcified cartilage and at the osteochondral junction. The boundary between osteophyte and articular cartilage was defined as the point where the tidemark or calcification front became indistinct, at which point collagen fibres in the cartilage developed a radially striated appearance (fig 1E,F). The length of the articular surface analysed was measured using digital electronic callipers (Mitutoyo, Kawasaki, Japan).
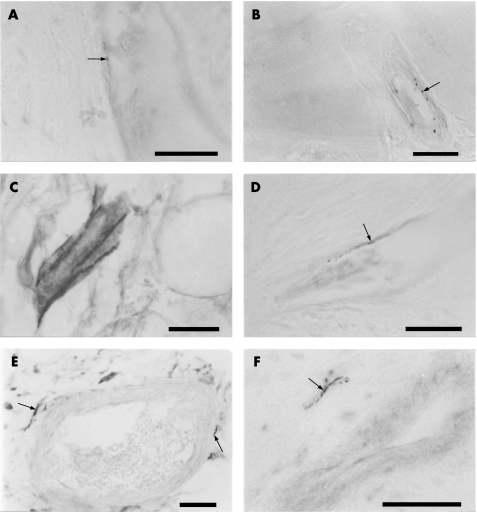
Figure 1 Neurovascular invasion of articular cartilage of osteoarthritic medial tibial plateaux. (A) Autofluorescence under 420 nm UV light showing blood vessels within channels in the non‐calcified cartilage, surrounded by a bony cuff. Scale bar = 200 μm. (B) Fluorescence under 615 nm UV light of the same section as (A) displaying CD34‐immunoreactive (red) vascular endothelium. Scale bar = 200 μm. (C) Transmitted light image of a haematoxylin and eosin‐stained section displaying a vascular channel crossing multiple tidemarks and entering the non‐calcified cartilage. The channel contains blood vessels, mononuclear cells and matrix, and is cuffed by bone. Scale bar = 100 μm. (D) Perivascular, protein gene product 9.5‐immunoreactive nerve fibre within a vascular channel crossing the tidemark. Scale bar = 100 μm. (E) Autofluorescence of bone, calcified cartilage and non‐calcified articular cartilage in the medial tibial plateau removed at total joint replacement from a patient with osteoarthritis. The cartilage has no striations and there is a clear tidemark. Scale bar = 200 μm. (F) Autofluorescence of cartilage in an osteophyte. The tidemark has been lost and the cartilage has a highly striated appearance. Scale bar = 200 μm. Empty arrowheads indicate tidemark, filled arrowheads indicate blood vessels, arrows indicate nerves, and asterisks indicate bone surrounding blood vessels.
Statistical analysis
Comparisons between groups were made using the Mann–Whitney U test, except for the comparisons of frequencies of neurovascular structures between femoral condyles and tibial plateaux, which were performed with Wilcoxon's test for paired data. Associations between continuous and categorical variables are reported as Spearman's rank correlation coefficients. Associations between dichotomous variables were determined by Fisher's exact test. Data are presented as median (range). p<0.05 was considered significant.
Materials
Rabbit antiserum to PGP 9.5 was from Chemicon International, Chandlers Ford, UK. Polyclonal CGRP antibody was from Peninsula Labs Inc, St Helens, UK. Rabbit polyclonal antisera to substance P and CPON were from Biomol, Exeter, UK. Mouse monoclonal antibody to CD34, clone QBEnd 10, was from Dako Cytomation, Ely, UK. Biotinylated affinity‐purified goat anti‐rabbit and horse anti‐mouse secondary antibodies, peroxidase‐labelled ABC peroxidase (ABC Elite) and alkaline phosphatase‐labelled ABC were from Vector Laboratories, Peterborough, UK. DPX mounting medium was from VWR Ltd, Lutterworth, UK. OCT compound was from Raymond Lamb, Eastbourne, UK. EDTA (sodium salt) was from Merck, Nottingham, UK. All other reagents were from Sigma Aldrich, Poole, UK unless otherwise stated.
Results
Autofluorescence, safranin O and haematoxylin and eosin stained sections revealed the expected structure of articular surfaces including calcified and non‐calcified cartilage separated by one or more tidemarks (fig 1). OA cartilage changes in the mid‐point of the medial tibial plateau were more severe in samples obtained at TKR (median score 9.5, range 5–13) than from samples obtained at PM (median score 5, range 2–10, p = 0.003). Samples from PM cases displayed mild to moderate OA cartilage changes, but no osteophytes. Clinical details were consistent with a diagnosis of early OA in PM case 3 (table 1). The absence of an accurate history of knee pain precluded diagnostic classification of OA in the remaining six PM cases, and severity of cartilage changes were taken as a surrogate index of OA.
Vascular channels entering the cartilage and breaching the tidemark originated from the subchondral bone (fig 1). Vascular channels were observed in both TKR and PM samples. In TKR samples, 23/37 (62%) and 16/37 (43%) displayed vessels entering the calcified cartilage and breaching the tidemark, respectively. In PM samples, 8/9 (89%) and 1/9 (11%) displayed vessels entering the calcified cartilage and breaching the tidemark, respectively (both p>0.05 compared with TKR). Endothelial cells, fibrovascular tissue, perivascular cells and red blood cells were observed in vascular channels. Often the vascular channels were surrounded by bone, and in some cases the bone extended to the tidemark (fig 1A–C). Blood vessels were also observed within the marrow spaces of osteophytes, and in subchondral bone (fig 2). The Mankin scores of samples with a nerve observed in the cartilage were not significantly different from those where nerves were not detected (Z = −1.1, p = 0.28). The frequency of neurovascular structures was not significantly different between articular cartilage in the tibial plateau and femoral condyle (Z = −0.68, p = 0.50).
Figure 2 Characterisation of nerves in osteoarthritic articular cartilage, osteophyte and subchondral bone. (A) Calcitonin gene‐related peptide‐immunoreactive (sensory) nerve fibre within a vascular channel that enters the calcified cartilage of the medial tibial plateau. (B) C‐flanking peptide of neuropeptide Y‐immunoreactive (sympathetic) nerve fibre within a vascular channel that enters the calcified cartilage of the medial tibial plateau. (C) Protein gene product 9.5‐immunoreactive nerve trunk within the bone marrow of a tibial osteophyte. (D) Calcitonin gene‐related peptide‐immunoreactive (sensory) nerve fibre within a tibial osteophyte. (E) Perivascular, protein gene product 9.5‐immunoreactive nerve fibres within the tibial subchondral bone. (F) C‐flanking peptide of neuropeptide Y‐immunoreactive (sympathetic) nerve fibre near a vessel within the tibial subchondral bone. Fine nerve fibres with a beaded morphology are indicated by arrows. All scale bars = 50 μm.
Nerves immunoreactive to PGP 9.5, substance P, CGRP and CPON were present in positive control synovium and in each of the four articular surfaces studied (medial tibial plateau, lateral tibial plateau, medial femoral condyle and lateral femoral condyle). Immunoreactive nerve fibres were demonstrated in articular cartilage and in subchondral bone in both TKR and PM samples, and also in osteophytes. Immunoreactive nerve fibres displayed a beaded morphology characteristic of nerve fibre terminals (figs 1D and 2A,B,D–F). Medial tibial plateaux, representing weight‐bearing compartments most affected by joint space narrowing in OA, contained both blood vessels and nerves, and were selected for more detailed immunohistochemical analysis.
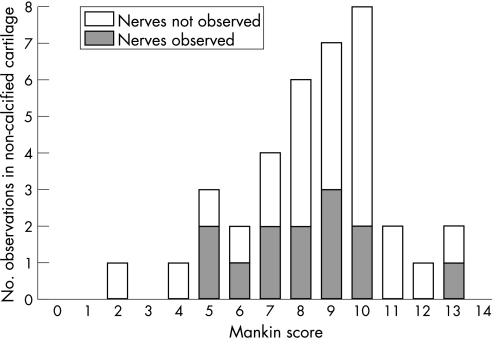
Nerve fibres immunoreactive to PGP 9.5, substance P, CGRP and CPON were each observed at the osteochondral junction in the articular cartilage of the medial tibial plateau (figs 1D, 2A,B). These nerves were always associated with blood vessels within vascular channels. Free nerves, not associated with blood vessels, were not observed within the articular cartilage. Correspondingly, innervation always accompanied vascular invasion at the osteochondral junction, although vessels were not always accompanied by detectable nerves (association between innervation and vascular breaching of tidemark, Fisher's exact test, p = 0.002). PGP 9.5‐immunoreactive nerves were observed within articular cartilage in 30% of cases. Nerves were observed within articular cartilage across most of the range of histological OA severity (fig 3; median Mankin score for samples containing nerves = 8, range 5–13). In samples where nerves were identified, the median density of nerves within articular cartilage was 12 (range 3–51) nerve profiles per mm2 of articular surface. Of cases that displayed PGP 9.5‐immunoreactive nerves, approximately equal proportions displayed sensory (immunoreactive to either substance P or CGRP; 43%) and sympathetic (42%) nerve fibres in the articular cartilage.
Figure 3 Innervation of the articular cartilage occurs across the range of Mankin scores in patients with osteoarthritis. Number of cases classified as either positive or negative for protein gene product 9.5‐immunoreactive nerves in the non‐calcified articular cartilage, plotted against their Mankin scores. Nerves were distributed across the range of Mankin scores, with similar ranges for both sensory (median = 8, range 5–10) and sympathetic (median = 6.5, range 5–9) nerves (not shown in figure).
Nerves were localised within the marrow cavities of osteophytes, both as individual nerve fibres and nerve trunks (fig 2C,D). Samples from seven (47%) of the 15 osteophytes studied displayed PGP 9.5 nerve fibre immunoreactivity. Samples from four (27%) of the 15 osteophytes were immunoreactive to sensory nerve markers (substance P or CGRP), and samples from six (40%) of the 15 osteophytes were immunoreactive to the sympathetic nerve marker, CPON. No differences in size, shape or distribution were observed between sensory and sympathetic nerves in osteophytes.
Perivascular and free nerve fibres, and nerve trunks were also observed within the subchondral bone marrow (fig 2E,F). PGP 9.5‐immunoreactive nerve fibres were found in the subchondral bone in 63% of OA cases compared with 33% of PM controls. Of cases that displayed PGP 9.5‐immunoreactive nerves, similar proportions displayed sensory (78%) and sympathetic nerve (64%) fibres within the subchondral bone.
Discussion
Vascularisation of the non‐calcified articular cartilage appears to be a common feature in human OA. The PM samples in this study displayed histological features of OA, with cartilage severity scores that overlapped with TKR samples. Whereas TKR samples were from patients with symptoms that were sufficiently severe to present for joint arthroplasty, the PM group probably includes patients with less advanced or preclinical stages of OA. Differentiating between early OA and age‐related changes, however, is extremely difficult, given the increasing prevalence of OA with age and the weak association between pathological changes and symptoms of OA. This may become possible if larger cohorts of normal samples and of cases of OA at a young age become available. Vascularisation of the non‐calcified cartilage was found throughout a wide range of histological OA severity, and was not restricted to end‐stage, surgical disease.
We have localised both sensory and sympathetic nerve fibres to the articular cartilage of human tibiofemoral OA. Innervation of the articular cartilage is therefore a potential source of pain in patients with knee OA. The exclusively perivascular localisation of nerves in the articular cartilage implies that vascularisation is the driving force behind its innervation. Similarly, innervation follows neovascularisation of skin grafts, subcutaneous sponge implants and in bony callus.20,21,26 As with vascularisation of the articular cartilage, innervation was not restricted to patients with end‐stage OA presenting for joint replacement surgery.
The number of nerves in the articular cartilage may be underestimated by our study because of limitations in the sensitivity of immunohistochemistry with existing antisera, fixation and decalcification techniques for human pathological samples. The small size of nerve fibres in relation to distances between fibres necessitated a painstaking systematic approach to analysis of immunohistochemical preparations of multiple thick tissue sections. Specific markers did not permit identification of sensory and sympathetic nerves in eight of the 13 PGP 9.5‐positive cases in this study. This indicates that cases may have been classified as negative for a particular neuronal marker when a further consecutive section may have revealed a nerve profile. Studies in small animals, in which perfusion fixation is possible, suggest higher densities of fine unmyelinated nerves in subchondral bone.27 Nonetheless, we have demonstrated that a substantial number of fine nerve terminals may be present in osteoarthritic cartilage.
We have shown sensory and sympathetic innervation of tibial osteophytes. Perivascular substance P‐immunoreactive nerve fibres have also been localised to the base of osteophytes in horse metacarpophalangeal OA.16 Sensory innervation of osteophytes may explain why radiological grading of osteophytosis is associated with reported pain severity.28 In contrast with our observations in man, rodent models have not displayed nerves in osteophytes.29,30 Rodent models of OA progress more quickly than human disease. Innervation is a slow process, and some months may be required to develop innervation.20,31 TKR samples in this study were all from patients with sufficient symptoms to indicate joint arthroplasty, although a more detailed assessment of pain severity could not be undertaken.
We found that the subchondral bone contains both sensory and sympathetic nerves, and therefore is also a potential source of tibiofemoral pain. Perivascular nerves have been demonstrated previously by the Bodian silver technique for axon staining in subchondral bone marrow from patients with hip OA.32 Although this technique was unable to distinguish sensory from sympathetic nerves, substance P‐immunoreactive sensory nerve fibres have been described in the subchondral bone of patellae and lumbar facet joints from patients with OA.18,33 Sensory nerves have also been reported in subchondral bone in murine OA,27 in normal horses,34,35 and in normal and arthritic rats.36,37,38
It is unclear whether subchondral sensory nerves display increased sensitivity or number in osteoarthritic joints.29 Our observed localisation of nerves within vascular channels and the marrow of osteophytes suggests that, in OA, nerves grow into these structures from subchondral bone, rather than from periosteum or synovium. Similarly, nerves invade callus from the adjacent marrow during bone repair.31
The mechanisms that drive innervation of osteoarticular tissues in OA remain incompletely understood. Our findings lead us to suppose that angiogenesis is required to permit innervation of articular cartilage. Inhibition of either nerve growth factors or angiogenesis may each offer potential to prevent the innervation of articular cartilage in OA. Such interventional studies will be required to elucidate the contribution of this innervation to osteoarthritic pain.
Acknowledgements
We are grateful to AstraZeneca UK for funding this research and the collection of the tissue repository. We are also grateful to all the patients, the orthopaedic surgeons and the Bereavement Centre at the Sherwood Forest Hospitals NHS Trust for providing clinical material. We also thank the histopathology personnel at the King's Mill Hospital for their help with the processing of tissue samples. DMcW is supported by arc grant 14851.
Abbreviations
TKR - total knee joint replacement
PM - post‐mortem
CPON - C‐flanking peptide of neuropeptide Y
CGRP - calcitonin gene‐related peptide
OA - osteoarthritis
PGP 9.5 - pan‐neuronal marker protein gene product 9.5
PBS - phosphate‐buffered saline
ABC - streptavidin–biotin complex
Footnotes
Competing interests: None.
References
- 1.Doherty M. Pathogenesis. Introduction: the concept of osteoarthritis as failure of the diarthrodial joint. In: Brandt KD, Doherty M, Lohmander S, eds. Osteoarthritis. 1st edn. Oxford: Oxford University Press 199870–74.
- 2.Clark J M. The structure of vascular channels in the subchondral plate. J Anat 1990171105–115. [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan H, Jundt J, Riddle J M, Pitchford W, Christopherson T. The tibial subchondral plate. A scanning electron microscopic study. J Bone Joint Surg [Am] 1987691212–1220. [PubMed] [Google Scholar]
- 4.Hashimoto S, Creighton‐Achermann L, Takahashi K, Amiel D, Coutts R D, Lotz M. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage 200210180–187. [DOI] [PubMed] [Google Scholar]
- 5.Gronblad M, Liesi P, Korkala O, Karaharju E, Polak J. Innervation of human bone periosteum by peptidergic nerves. Anat Rec 1984209297–299. [DOI] [PubMed] [Google Scholar]
- 6.Arnoldi C C, Lemperg K, Linderholm H. Intraosseous hypertension and pain in the knee. J Bone Joint Surg [Br] 197557360–363. [PubMed] [Google Scholar]
- 7.Gronblad M, Korkala O, Liesi P, Karaharju E. Innervation of synovial membrane and meniscus. Acta Orthop Scand 198556484–486. [DOI] [PubMed] [Google Scholar]
- 8.Dye S F, Vaupel G L, Dye C C. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med 199826773–777. [DOI] [PubMed] [Google Scholar]
- 9.Wagstaff S, Smith O V, Wood P H. Verbal pain descriptors used by patients with arthritis. Ann Rheum Dis 198544262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gronblad M, Konttinen Y T, Korkala O, Liesi P, Hukkanen M, Polak J M. Neuropeptides in synovium of patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 1988151807–1810. [PubMed] [Google Scholar]
- 11.Hirasawa Y, Okajima S, Ohta M, Tokioka T. Nerve distribution to the human knee joint: anatomical and immunohistochemical study. Int Orthop 2000241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanzetta A, Corradini C, Verdoia C, Miani A, Castano S, Castano P. The nervous structures of anterior cruciate ligament of human knee, healthy and lesioned, studied with confocal scanning laser microscopy. Ital J Anat Embryol 2004109167–176. [PubMed] [Google Scholar]
- 13.McDougall J J, Bray R C, Sharkey K A. Morphological and immunohistochemical examination of nerves in normal and injured collateral ligaments of rat, rabbit, and human knee joints. Anat Rec 199724829–39. [DOI] [PubMed] [Google Scholar]
- 14.Mine T, Kimura M, Sakka A, Kawai S. Innervation of nociceptors in the menisci of the knee joint: an immunohistochemical study. Arch Orthop Trauma Surg 2000120201–204. [DOI] [PubMed] [Google Scholar]
- 15.Witonski D, Wagrowska‐Danilewicz M. Distribution of substance‐P nerve fibers in intact and ruptured human anterior cruciate ligament: a semi‐quantitative immunohistochemical assessment. Knee Surg Sports Traumatol Arthrosc 200412497–502. [DOI] [PubMed] [Google Scholar]
- 16.Wojtys E M, Beaman D N, Glover R A, Janda D. Innervation of the human knee joint by substance‐P fibers. Arthroscopy 19906254–263. [DOI] [PubMed] [Google Scholar]
- 17.Fortier L A, Nixon A J. Distributional changes in substance P nociceptive fiber patterns in naturally osteoarthritic articulations. J Rheumatol 199724524–530. [PubMed] [Google Scholar]
- 18.Beaman D N, Graziano G P, Glover R A, Wojtys E M, Chang V. Substance P innervation of lumbar spine facet joints. Spine 1993181044–1049. [DOI] [PubMed] [Google Scholar]
- 19.Brown M F, Hukkanen M V, McCarthy I D, Redfern D R, Batten J J, Crock H V.et al Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg [Br] 199779147–153. [DOI] [PubMed] [Google Scholar]
- 20.Walsh D A, Hu D E, Mapp P I, Polak J M, Blake D R, Fan T P. Innervation and neurokinin receptors during angiogenesis in the rat sponge granuloma. Histochem J 199628759–769. [DOI] [PubMed] [Google Scholar]
- 21.Aoki M, Tamai K, Saotome K. Substance P‐ and calcitonin gene‐related peptide‐immunofluorescent nerves in the repair of experimental bone defects. Int Orthop 199418317–324. [DOI] [PubMed] [Google Scholar]
- 22.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K.et al Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986291039–1049. [DOI] [PubMed] [Google Scholar]
- 23.Stefanini M, De Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature 1967216173–174. [DOI] [PubMed] [Google Scholar]
- 24.Mankin H J, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo‐arthritic human hips. J Bone Joint Surg [Am] 197052424–434. [PubMed] [Google Scholar]
- 25.Shu S Y, Ju G, Fan L Z. The glucose oxidase‐DAB‐nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett 198885169–171. [DOI] [PubMed] [Google Scholar]
- 26.Kangesu T, Manek S, Terenghi G, Gu X H, Navsaria H A, Polak J M.et al Nerve and blood vessel growth in response to grafted dermis and cultured keratinocytes. Plast Reconstr Surg 19981011029–1038. [DOI] [PubMed] [Google Scholar]
- 27.Mach D B, Rogers S D, Sabino M C, Luger N M, Schwei M J, Pomonis J D.et al Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 2002113155–166. [DOI] [PubMed] [Google Scholar]
- 28.Neame R L, Carr A J, Muir K, Doherty M. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis 200362513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buma P, Verschuren C, Versleyen D, Van der Kraan P, Oestreicher A B. Calcitonin gene‐related peptide, substance P and GAP‐43/B‐50 immunoreactivity in the normal and arthrotic knee joint of the mouse. Histochemistry 199298327–339. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Nagata K, Iijima T. Involvement of sensory nerves and immune cells in osteophyte formation in the ankle joint of adjuvant arthritic rats. Histochem Cell Biol 2002118213–220. [DOI] [PubMed] [Google Scholar]
- 31.Madsen J E, Hukkanen M, Aspenberg P, Polak J, Nordsletten L. Time‐dependent sensory nerve ingrowth into a bone conduction chamber. Acta Orthop Scand 20007174–79. [DOI] [PubMed] [Google Scholar]
- 32.Reimann I, Christensen S B. A histological demonstration of nerves in subchondral bone. Acta Orthop Scand 197748345–352. [DOI] [PubMed] [Google Scholar]
- 33.Kallakuri S, Singh A, Chen C, Cavanaugh J M. Demonstration of substance P, calcitonin gene‐related peptide, and protein gene product 9.5 containing nerve fibers in human cervical facet joint capsules. Spine 2004291182–1186. [DOI] [PubMed] [Google Scholar]
- 34.Bowker R M, Rockershouser S J, Linder K, Vex K B, Sonea I M, Caron J P. A silver‐impregnation and immunocytochemical study of innervation of the distal sesamoid bone and its suspensory ligaments in the horse. Equine Vet J 199426212–219. [DOI] [PubMed] [Google Scholar]
- 35.Nixon A J, Cummings J F. Substance P immunohistochemical study of the sensory innervation of normal subchondral bone in the equine metacarpophalangeal joint. Am J Vet Res 19945528–33. [PubMed] [Google Scholar]
- 36.Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M. Substance P‐ and CGRP‐immunoreactive nerves in bone. Peptides 19889165–171. [DOI] [PubMed] [Google Scholar]
- 37.Hukkanen M, Konttinen Y T, Rees R G, Gibson S J, Santavirta S, Polak J M. Innervation of bone from healthy and arthritic rats by substance P and calcitonin gene related peptide containing sensory fibers. J Rheumatol 1992191252–1259. [PubMed] [Google Scholar]
- 38.Imai S, Tokunaga Y, Maeda T, Kikkawa M, Hukuda S. Calcitonin gene‐related peptide, substance P, and tyrosine hydroxylase‐immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J Orthop Res 199715133–140. [DOI] [PubMed] [Google Scholar]